* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Chapter 2 Power Point
Ceramic engineering wikipedia , lookup
Spinodal decomposition wikipedia , lookup
Abundance of the chemical elements wikipedia , lookup
History of molecular theory wikipedia , lookup
Thermomechanical analysis wikipedia , lookup
California Green Chemistry Initiative wikipedia , lookup
Chemical element wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Photopolymer wikipedia , lookup
Chemistry: A Volatile History wikipedia , lookup
Al-Shifa pharmaceutical factory wikipedia , lookup
Inorganic chemistry wikipedia , lookup
Organic chemistry wikipedia , lookup
IUPAC nomenclature of inorganic chemistry 2005 wikipedia , lookup
Gas chromatography wikipedia , lookup
Chemical industry wikipedia , lookup
Chemical weapon proliferation wikipedia , lookup
Stoichiometry wikipedia , lookup
Chemical potential wikipedia , lookup
Chemical weapon wikipedia , lookup
Atomic theory wikipedia , lookup
Chemical plant wikipedia , lookup
Chemical Corps wikipedia , lookup
Vapor–liquid equilibrium wikipedia , lookup
Condensed matter physics wikipedia , lookup
Gas chromatography–mass spectrometry wikipedia , lookup
Drug discovery wikipedia , lookup
History of chemistry wikipedia , lookup
Registration, Evaluation, Authorisation and Restriction of Chemicals wikipedia , lookup
Chemical thermodynamics wikipedia , lookup
Safety data sheet wikipedia , lookup
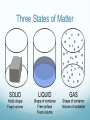
Unit 1 Chapter 2-pg38 Matter and Change Chemistry 334 Essential Question: How do chemists classify matter? Section 2.1 Properties of Matter Describing Matter KC: How can Properties be used to describe matter? A: Properties used to describe matter can be classified as ether extensive or intensive. Extensive Properties A property that depends on the amount of matter in a sample. Examples of extensive properties: Mass—the amount of matter an object contains. Volume—the measure of the space occupied by an object. Intensive Properties: A property that depends on the type of matter in a sample, not the amount. Examples Hardness Odor Conductivity State of matter *******What it is made of****** Identifying Substances KC: Why do all samples of a substance have the same intensive properties? Every sample of a given substance has identical intensive properties because every sample has the same composition. Substance Matter that has definite and uniform composition. AKA: Pure substance Examples: Au Ag NaCl CH4 NaCl vs. NaCl Water (H2O) Q: Is salt water a pure substance???? INDEX CARD CHALLENGE!!!! INDEX CARD CHALLENGE Without talking, find someone who has a different answer that you IDK—does not count as an answer!!! Explain why you chose this answer. The Answer NaCl is a pure substance Salt water is not It can have variable composition Physical Properties Physical property—any quality or condition of a substance that can be observed without changing the substance’s composition. Examples: State Mp Bp Color States of matter: KC: What are 3 states of matter? A: Three states of matter are solid, liquid, gas. Solid Definite shape Definite volume Not easily compressed Liquid Indefinite shape Definite volume Not easily compressed Gas Indefinite shape Indefinite volume Easily compressed Three States of Matter Vapor Describes the gaseous state of a substance that is generally a liquid or solid at room temperature. Physical Changes KC: How can physical changes be classified? A: Physical changes can be classified as reversible or irreversible. Reversible Phase changes Melting Freezing Sublimation Boiling Condensation Irreversible Breaking Tearing Smashing Cutting Section 2.2 Mixtures Page 44 Classifying Mixtures KC: How can mixtures be classified? A: Mixtures can be calssified as either hetterogenous or homogenous. Mixture A combination of two or more pure substance. Can be made with different combinations of solid, liquids and gasses. 2 Types of mixtures 1. Homogenous 2. Heterogenous Heterogeneous Mixture Not the same through out More than one phase (distinguishable parts) ie: Chicken noodle soup Chex mix Homogenous mixture Uniform composition; same through out AKA: Solution Only one phase ie: Salt water Tea Olive oil Vinegar Alloy Separating Mixtures KC: How can mixtures be seperated? A: Differences in physical properties can be used to separate mixtures. 5 ways to separate mixtures 1. Filtration 2. Distillation 3. Crystallization 4. Sublimation 5. Chromatography Filtration A technique which uses a pours barrier to separate a solid from a liquid Distillation A separation technique that is based on the differences in the boiling points of the mixed substances Crystallization A separation technique that results in the formation of pure solid particles of a substance from a solution containing the dissolved substance. Sublimation The process during which a solid changes to a vapor without going through the liquid phase Chromatography A technique that separates the components of a mixture (aka-mobile phase), based on the ability of each component to be drawn across the surface of another material (aka-stationary phase). Section 2.3 Elements and Compounds Distinguishing Elements and Compounds KC: How are elements and compounds different? A: Compounds can be broken down into simpler substances. Examples of elements Ca Na H Periodic Table Organizes the elements into a grid of horizontal rows (aka-periods) and vertical columns (akagroups/families). Compounds Two or more elements combined chemically Breaking Down Compounds Compounds can be broken down by chemical change This process usually requires a an external energy source, such as heat or electricity Properties of Compounds The chemical and physical characteristics of a compound is very different than that of its constitute parts. Example: K + I KI Potassium & Iodine VS Potassium iodide Potassium A light silver metal that reacts with water Iodine A black solid that changes to a purple gas at room temperature. Potassium iodide A white salt Distinguishing Substances and Mixtures KC: How can substances and mixtures be distinguished? A: If the composition of a material is fixed, the material is a substance. If the composition of the substance may vary, the material is a mixture. Classification of matter Ex: chex mix salt water (AKA: solutions) NaCl Na Symbols and formulas KC: What do chamists use to represent elements and compounds? A: Chemists use chemical symbols to repersent elenents and chemical formulas to represent compounds Chemical Symbol One or two letters used to represent the name of an element. Chemical Compounds Section 2.4 Chemical Reactions Chemical Changes Key Concept: What always happens during a chemical change? Answer: During a chemical change, the composition of matter changes Example combustion reaction Examples: Fe to Rust Respiration Combustion (candle burning vs. wax melting) Words that indicate a chemical change: -Decompose -Ferment -Tarnish -Oxidize -Burn -Rust -Corrode -Rot -Explode Chemical Properties= The ability (or inability) of a substance to combine with or change into one or more substances. Chemical Change One or more substances changes into one or more new substances. AKA: Chemical reaction Mg + HCl H2 + MgCl Reactants The substance that are to be transformed. Product The substance(s) that are made Recognizing Chemical Changes Key Concept: What are the 4 possible clues that a chemical change has taken place? Answer: Possible clues that a chemical reaction has taken place include: transfer of energy (heat or light), a change in color, the production of gas, or the formation of a precipitate.` Precipitate= The production of a solid. Conservation of Mass Key concept: How are the masses of the reactants and the masses of the products of a reaction related? Answer: During any chemical reaction the mass of the reactants is always equal to the mass of the products. Law of Conservation of Mass: Massreactants=Massproducts Practice Problems 2Mg + O2 2 MgO Mass of Magnesium (g) Mass of Oxygen (g) Mass of Magnesium oxide (g) 5 3.3 8.3 6.5 A 10.8 13.6 9 B C 12.5 31.5 Words that indicate a chemical change: -Decompose -Ferment -Tarnish -Oxidize -Burn -Rust -Corrode -Rot -Explode Physical Changes= A change which alters a substance without changing its composition. Examples of physical changes: Cutting Braking Phase Change=transition of mater from one state to another. Chemical changes vs. physical changes Physical Changes Alters a substance without Chemical Changes Alters a substance by changing its composition (arrangement of its atoms) changing its composition (arrangement of its atoms) Does NOT change the arrangement of atoms DOES change the arrangement of atoms SAME substance! NEW substance made! Chemical/Physical changes Foldable