* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download 13 Physiologicoanatomical peculiarities of endocrine system in
Survey
Document related concepts
Menstrual cycle wikipedia , lookup
Neuroendocrine tumor wikipedia , lookup
Mammary gland wikipedia , lookup
Bioidentical hormone replacement therapy wikipedia , lookup
Cryptorchidism wikipedia , lookup
Breast development wikipedia , lookup
Hormone replacement therapy (male-to-female) wikipedia , lookup
Xenoestrogen wikipedia , lookup
Endocrine disruptor wikipedia , lookup
Adrenal gland wikipedia , lookup
Hyperandrogenism wikipedia , lookup
Graves' disease wikipedia , lookup
Transcript
Physiologic anatomical
peculiarities of endocrine
system in children. Methodics of
endocrine glands investigation.
Semiotics of hypo- and
hyperfunction of some
endocrine glands and diseases
of the endocrine system.
By Nykytyuk S
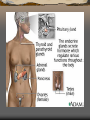
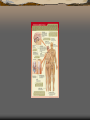
Major endocrine glands.
(Male left, female on the
right.)
1. Pineal gland
2. Pituitary gland
3. Thyroid gland
4. Thymus
5. Adrenal gland
6. Pancreas
7. Ovary
8. Testis
The endocrine system provides a chemical connection from the
hypothalamus of the brain to all the organs that control body
metabolism, growth and development, and reproduction.
There are two types of hormones secreted in the endocrine
system: (1) steroidal and (2) nonsteroidal, or protein based,
hormones. The endocrine system regulates its hormones through
negative feedback control. Increases in hormone activity
decreases the production of that hormone. The immune system
and other factors contribute as control factors also, maintaining
constant levels of hormones.
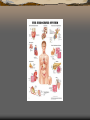
Endocrine glands and the hormones
secreted
1.
2.
3.
4.
5.
6.
Hypothalamus produces
Thyrotropin-releasing hormone
(TRH)
Gonadotropin-releasing hormone
(GnRH)
Growth hormone-releasing
hormone (GHRH)
Corticotropin-releasing hormone
(CRH)
Somatostatin (SS; also GHIH,
growth factor-inhibiting hormone)
Dopamine (DA)
1.
Pineal Gland produces
Melatonin
Endocrine glands and the hormones
secreted
Pituitary gland (hypophysis)
produces
Anterior pituitary lobe
(adenohypophysis)
Growth hormone (GH)
Prolactin (PRL)
Adrenocorticotropic hormone
(ACTH, corticotropin)
Thyroid-stimulating hormone
(TSH, thyrotropin)
Follicle-stimulating hormone (FSH,
a gonadotropin)
Luteinizing hormone (LH, a
gonadotropin)
1.
2.
3.
Posterior pituitary lobe
(neurohypophysis)
Oxytocin (ocytocin)
Arginine vasopressin (AVP; also
ADH, antidiuretic hormone)
Lipotropin
Endocrine glands and the hormones
secreted
Thyroid gland produces
Triiodothyronine (T3), the
potent form of thyroid hormone
Thyroxine (T4), a less active
form of thyroid hormone
Calcitonin
Parathyroid gland produces
Parathyroid hormone (PTH)
Heart produces
Atrial-natriuretic peptide (ANP)
Stomach and intestines
produce
Cholecystokinin (CCK)
Gastrin
Ghrelin
Neuropeptide Y (NPY)
Secretin
Somatostatin
Endocrine glands and the hormones
secreted
Liver produces
Insulin-like growth factor (IGF)
Angiotensinogen
Thrombopoietin
Glucocorticoids (chiefly
cortisol)
Mineralocorticoids (chiefly
aldosterone)
Androgens (including DHEA
and testosterone)
Islets of Langerhans in the
pancreas produce
Insulin
Glucagon
Somatostatin
Adrenal glands produce
Adrenal cortex
Adrenal medulla
Adrenaline (epinephrine)
Noradrenaline
(norepinephrine)
Testosterone
Endocrine glands and the hormones
secreted
Kidney produces
Renin
Erythropoietin (EPO)
Calcitriol (the active form of vitamin D3)
Skin produces
Vitamin D3 (calciferol)
Adipose tissue
Leptin
Estrogens (mainly estrone
Endocrine glands and the hormones
secreted
In males only
Testes
Androgens (chiefly
testosterone)
In females only
Ovarian follicle
Estrogens (mainly estradiol)
Corpus luteum
Progesterone
Estrogens (mainly estradiol)
Placenta (when pregnant)
Progesterone
Estrogens (mainly estriol)
Human chorionic gonadotropin
(HCG)
Human placental lactogen
(HPL)
Pineal gland
The pineal gland is a
reddish-gray body about the
size of a pea (8 mm in
humans), located just rostrodorsal to the superior
colliculus and behind and
beneath the stria medullaris,
between the laterally
positioned thalamic bodies. It
is part of the epithalamus.
The pineal gland is large in children, but shrinks at
puberty. It appears to play a major role in sexual
development, hibernation in animals, metabolism, and
seasonal breeding. The abundant melatonin levels in
children is believed to inhibit sexual development, and
pineal tumors have been linked with precocious puberty.
When puberty arrives, melatonin production is reduced.
Calcification of the pineal gland is typical in adults.
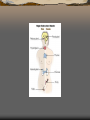
Pituitary gland
The pituitary gland, or
hypophysis, is an endocrine gland
about the size of a pea that sits in a
small, bony cavity (sella turcica) at
the base of the brain.
The pituitary gland secretes
hormones regulating homeostasis,
including trophic hormones that
stimulate other endocrine glands. It
is functionally connected to the
hypothalamus by the median
eminence.
Posterior pituitary
(neurohypophysis)
The posterior lobe is connected to a part of the brain called the
hypothalamus via the infundibulum (or stalk), giving rise to the
tuberoinfundibular pathway. Hormones are made in nerve cell
bodies positioned in the hypothalamus, and these hormones are
then transported down the nerve cell's axons to the posterior
pituitary. Hypothalamic neurons fire such hormones, releasing
them into the capillaries of the pituitary gland.
The hormones secreted by the posterior pituitary are
Oxytocin comes from the paraventricular nucleus in the
Hypothalamus
Antidiuretic hormone (ADH - also known as vasopressin), comes
from the supraoptic nucleus in the Hypothalamus
Anterior pituitary
(Adenohypophysis)
1.
2.
3.
4.
5.
6.
The anterior pituitary produces and
secretes:
growth hormone
prolactin
follicle-stimulating hormone
luteinizing hormone
thyroid-stimulating hormone
adrenocorticotropic hormone
endorphins
and other hormones
It does this in response to releasing
hormones produced by the
hypothalamus. These travel to the
anterior lobe by way of a special
capillary system, called the
hypothalamic-hypophyseal portal
system. These hypothalamic signalling
hormones include:
TRH (thyrotropin-releasing hormone)
CRH (corticotropin-releasing hormone)
DA (dopamine, "prolactin inhibiting
factor"/PIF)
GnRH (gonadotropin-releasing
hormone)
GHRH (growth hormone releasing
hormone)
Intermediate lobe
In adult humans it is just a thin layer of cells
between the anterior and posterior pituitary,
nearly indistinguishable from the anterior lobe.
The intermediate lobe produces melanocytestimulating hormone (MSH), although this
function is often (imprecisely) attributed to the
anterior pituitary.
Functions
The pituitary gland helps control the following body processes:
1. Growth
2. Blood pressure
3. Some aspects of pregnancy and childbirth
4. Breast milk production
5. Sex organ functions in both women and men
6. Thyroid gland function
7. The conversion of food into energy (metabolism)
8. Water and osmolarity regulation in the body
Adrenocorticotropic hormone
ACTH acts through the stimulation of cell surface ACTH
receptors, which are primarily located on the
adrenocortical cells. ACTH stimulates the cortex of the
adrenal gland and boosts the synthesis of
corticosteroids, mainly glucocorticoids but also
mineralcorticoids and sex steroids (androgens).
Together with ACTH the hormones lipotropin,
melanocyte-stimulating hormone (MSH), β-endorphin
and met-enkephalin are also released. ACTH is also
related to the circadian rhythm in many organisms.
Growth hormone
Growth hormone (GH
or somatotropin) is a
polypeptide hormone
synthesised and
secreted by the anterior
pituitary gland which
stimulates growth and
cell reproduction in
humans
Functions of GH
Effects of growth hormone on the tissues of the body can
generally be described as anabolic (building up). Like most other
protein hormones GH acts by interacting with a specific receptor
on the surface of cells.
Height growth in childhood is the best known effect of GH action,
and appears to be stimulated by at least two mechanisms.
1. GH directly stimulates division and multiplication of
chondrocytes of cartilage. These are the primary cells in the
growing ends (epiphyses) of children's long bones (arms, legs,
digits).
2. GH also stimulates production of insulin-like growth factor 1
(IGF1, formerly known as somatomedin C), a hormone
homologous to proinsulin.
Growth hormone excess: (acromegaly and pituitary
gigantism)
The most common disease of GH excess is a pituitary tumor
comprised of somatotroph cells of the anterior pituitary. These
somatotroph adenomas are benign and grow slowly, gradually
producing more and more GH. Prolonged GH excess thickens
the bones of the jaw, fingers and toes. Resulting heaviness of the
jaw and increased thickness of digits is referred to as
acromegaly. GH-secreting tumors are typically recognized in the
5th decade of life. It is extremely rare for such a tumor to occur in
childhood, but when it does the excessive GH can cause
excessive growth, traditionally referred to as pituitary gigantism.
Growth hormone deficiency (GHD)
Deficiency of GH produces significantly different
problems at various ages. In children, growth
failure and short stature are the major
manifestations of GH deficiency. In adults the
effects of deficiency are more subtle, and may
include deficiencies of strength, energy, and
bone mass, as well as increased cardiovascular
risk.
Other GH uses and treatment indications
Many other conditions besides GH deficiency cause
poor growth, but growth benefits (height gains) are often
poorer than when GH deficiency is treated. Examples of
other causes of shortness often treated with growth
hormone are Turner syndrome, chronic renal failure,
Prader-Willi syndrome, intrauterine growth retardation,
and severe idiopathic short stature. Higher
("pharmacologic") doses are required to produce
significant acceleration of growth in these conditions,
producing blood levels well above physiologic.
Thyroid-stimulating hormone
Thyroid-stimulating hormone (also known as TSH or
thyrotropin) is a hormone synthesized and secreted by
thyrotrope cells in the anterior pituitary gland which regulates the
endocrine function of the thyroid gland. TSH stimulates the
thyroid gland to secrete the hormones thyroxine (T4) and
triiodothyronine (T3). TSH production is controlled by a
Thyrotropin Releasing Hormone, (TRH), which is manufactured
in the hypothalamus and transported to the pituitary gland, where
it increases TSH production and release. Somatostatin is also
produced by the hypothalamus, and has an opposite effect on
the pituitary production of TSH, decreasing or inhibiting its
release.
Primarily Abnormal Pituitary Function
Higher than normal levels of TSH combined with high
levels of thyroid hormone (T3 and T4) may indicate
dysfunction of the hypothalamus and pituitary gland. In
these case, a high TSH is often produced by a benign
tumor of the pituitary (adenoma). Conversely, low levels
of TSH, while blood levels of T3 and T4 are also low,
indicates abnormally low function of the pituitary, known
as hypopituitarism.
Primarily Abnormal Thyroid function
On the other hand, due to the negative feedback
described above, abnormally high levels of Thyroid
hormone, due to overproduction in the thyroid, results in
low TSH levels. This occurs in diseases such as
hyperthyroidism or Grave's disease. Conversely, an
underproduction of T3 and T4 caused by diseases such
as congenital hypothyroidism (cretinism),
hypothyroidism or thyroid hormone resistance, gives
rise to an increase in the measured TSH.
Clearly both TSH and T3 and T4 should be
measured to ascertain where a specific thyroid
disfunction is caused by primary pituitary or by a
primary thyroid disease. If both are up (or down)
then the problem is probably in the pituitary. If
the one component (TSH) is up, and the other
(T3 and T4) is down, then the disease is
probably in the thyroid itself. The same holds for
a low TSH, high T3 and T4 finding.
Prolactin
Prolactin is a peptide hormone synthesised and
secreted by lactotrope cells in the adenohypophysis
(anterior pituitary gland). It is also produced in other
tissues including the breast and the decidua. Pituitary
prolactin secretion is regulated by neuroendocrine
neurons in the hypothalamus, most importantly by
neurosecretory dopamine neurons of the arcuate
nucleus, which inhibit prolactin secretion.
Prolactin has many effects, the
most important of which is to
stimulate the mammary glands to
produce milk (lactation).
Increased serum concentrations of
prolactin during pregnancy cause
enlargement of the mammary
glands of the breasts and
increases the production of milk.
However, the high levels of
progesterone during pregnancy act
directly on the breasts to stop
ejection of milk. It is only when the
levels of this hormone fall after
childbirth that milk ejection is
possible.
Follicle-stimulating hormone
Follicle stimulating hormone (FSH) is a hormone
synthesised and secreted by gonadotropes in the
anterior pituitary gland. In the ovary FSH stimulates the
growth of immature Graafian follicles to maturation. As
the follicle grows it releases inhibin, which shuts off the
FSH production. In men, FSH enhances the production
of androgen-binding protein by the Sertoli cells of the
testes and is critical for spermatogenesis. FSH and LH
act synergistically in reproduction.
High FSH levels
High FSH levels are indicative of situations where the normal
restricting feedback from the gonad is absent, leading to an
unrestriced pituitary FSH production. While this is typical in the
menopause, it is abnormal in the reproductive years. There it
may be a sign of:
1. Premature menopause
2. Gonadal dysgenesis, Turner syndrome
3. Castration
4. Swyer syndrome
5. Certain forms of CAH
6. Testicular failure
Deficient FSH activity
1.
2.
3.
4.
5.
6.
Diminished secretion of FSH can result in failure of gonadal function
(hypogonadism). This condition is typically manifest in males as failure in
production of normal numbers of sperm. In females, cessation of reproductive
cycles is commonly observed. Conditions with very low FSH secretions are:
Kallmann syndrome
Hypothalamic suppression
Hypopituitarism
Hyperprolactinemia
Gonadotropin deficiency
Gonadal suppression therapy
GnRH antagonist
GnRH agonist (downregulation)
Luteinizing hormone
Luteinizing hormone (LH) is a hormone synthesized
and secreted by gonadotropes in the anterior lobe of the
pituitary gland. In concert with the other pituitary
gonadotropin follicle stimulating hormone (FSH) it is
necessary for proper reproductive function. In the
female, an acute rise of LH – the LH surge – triggers
ovulation. In the male, where LH had also been called
Interstitial Cell Stimulating Hormone (ICSH), it
stimulates Leydig cell production of testosterone.
LH levels are normally low during childhood and,
in women, high after menopause. During the
reproductive years typical levels are seen
between 5-20 mIU/ml. Physiologic high LH levels
are seen during the LH surge (v.s.), typically they
last 48 hours.
Disease States
Relative elevations
In children with precocious puberty of
pituitary or central origin, LH and FSH
levels may be in the reproductive range
and not at the low levels typically for
their age.
High LH levels
Persistently high LH levels are
indicative of situations where the
normal restricting feedback from the
gonad is absent, leading to an
unrestricted pituitary production of
both, LH and FSH. While this is typical
in the menopause, it is abnormal in the
reproductive years. There it may be a
sign of:
1. Premature menopause
2. Gonadal dysgenesis, Turner syndrome
3. Castration
4. Swyer syndrome
5. Certain forms of CAH
6. Testicular failure
Deficient LH activity
Diminished secretion of LH can result in failure of gonadal
function (hypogonadism). This condition is typically manifest in
males as failure in production of normal numbers of sperm. In
females, amenorrhea is commonly observed. Conditions with
very low FSH secretions are:
1. Kallmann syndrome
2. Hypothalamic suppression
3. Hypopituitarism
4. Eating disorder
5. Hyperprolactinemia
6. Gonadotropin deficiency
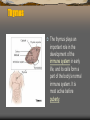
Thymus
The thymus plays an
important role in the
development of the
immune system in early
life, and its cells form a
part of the body's normal
immune system. It is
most active before
puberty.
In the two thymic lobes, lymphocyte precursors mature
into T cells (where T stands for “thymus”). The thymus
is critically required for the production of the vast
majority of T cells. Once made, T cells leave the thymus
and patrol the body.
They protect against foreign invaders by making
immune responses, that are initiated via T cell receptors
expressed by these T cells. Each T cell has a different T
cell receptor, allowing the immune system to recognize
many distinct foreign invaders by generating many T
cells.
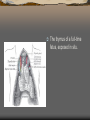
The thymus of a full-time
fetus, exposed in situ.
Immature thymocytes undergo a
process of selection, based on the
specificity of their T cell receptors.
This involves selection of T cells
that are functional (positive
selection), and elimination of T
cells that are autoreactive
(negative selection).
Cells that pass both levels of
selection are released into the
bloodstream to perform vital
immune functions.
Thymus continues to grow until the time of puberty and then
begins to atrophy.
The thymus is most active before puberty, after which it shrinks
in size and activity in most individuals and is replaced with fat (a
phenomenon known as "involution").
1. birth-about 15 grams;
2. Puberty-about 35 grams
3. twenty-five years-25 grams
4. sixty years-less than 15 grams
5. seventy years-about 6 grams
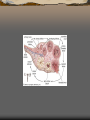
Pancreas
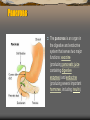
The pancreas is an organ in
the digestive and endocrine
system that serves two major
functions: exocrine
(producing pancreatic juice
containing digestive
enzymes) and endocrine
(producing several important
hormones, including insulin).
1: Head of pancreas
2: Uncinate process of pancreas
3: Pancreatic notch
4: Body of pancreas
5: Anterior surface of pancreas
6: Inferior surface of pancreas
7: Superior margin of pancreas
8: Anterior margin of pancreas
9: Inferior margin of pancreas
10: Omental tuber
11: Tail of pancreas
12: Duodenum
There are four main types of cells in
the islets of Langerhans.
beta cells-Insulin and
Amylin
alpha cells-Glucagon
Deltacells-Somatostatin
PP cells-Pancreatic
polypeptide
50-80% lower blood
sugar
15-20%raise blood sugar
3-10%inhibit endocrine
pancreas
1%inhibit exocrine
pancreas
Insulin
The structure of insulin. The lefthand side is a space-filling model
of the insulin monomer, believed to
be biologically active. Carbon is
green, hydrogen white, oxygen red,
and nitrogen blue. On the righthand side is a cartoon of the
hexamer, believed to be the stored
form. A monomer unit is highlighted
with the A chain in blue and the B
chain in cyan. Yellow denotes
disulfide bonds, and magenta
spheres are zinc ions.
Computer-generated
image of insulin
hexamers highlighting
the threefold symmetry,
the zinc ions holding it
together, and the
histidine residues
involved in zinc
binding.
Insulin (from Latin insula, "island",
as it is produced in the Islets of
Langerhans in the pancreas) is a
polypeptide hormone that regulates
carbohydrate metabolism. Apart
from being the primary effector in
carbohydrate homeostasis, it has
effects on fat metabolism and it can
change the liver's ability to release
fat stores. Insulin's concentration
has extremely widespread effects
throughout the body.
Nobel Prizes
Macleod and Banting were awarded the Nobel Prize in Physiology or Medicine
in 1923 for the discovery of insulin. Banting, insulted that Best was not
mentioned, shared his prize with Best, and MacLeod immediately shared his
with Collip. The patent for insulin was sold to the University of Toronto for one
dollar.
The exact sequence of amino acids comprising the insulin molecule, the socalled primary structure, was determined by British molecular biologist
Frederick Sanger. It was the first protein to have its structure be completely
determined. He was awarded the Nobel Prize in Chemistry in 1958.
In 1967, after decades of work, Dorothy Crowfoot Hodgkin determined the
spatial conformation of the molecule, by means of X-ray diffraction studies.
She had been awarded a Nobel Prize in Chemistry in 1964 for the
development of crystallography.
Rosalyn Sussman Yalow received the 1977 Nobel Prize in Medicine for the
development of the radioimmunoassay for insulin.
Glucose test
Diabetes mellitus
Diabetes mellitus is a metabolic disorder, specifically
affecting carbohydrate metabolism. It is a disease
characterized by persistent hyperglycemia (high
glucose blood sugar). It is a metabolic disease that
requires medical diagnosis, treatment and lifestyle
changes. The World Health Organization recognizes
three main forms of diabetes: type 1, type 2 and
gestational diabetes (or type 3, occurring during
pregnancy)[1], although these three "types" of diabetes
are more accurately considered patterns of pancreatic
failure rather than single diseases.
Type 1 diabetes mellitus
Type 1 diabetes mellitus - formerly known as insulindependent diabetes (IDDM), childhood diabetes, or
juvenile-onset diabetes - is characterized by loss of the
insulin-producing beta cells of the islets of Langerhans
of the pancreas leading to a deficiency of insulin. It
should be noted that there is no known preventative
measure which can be taken to avoid type 1 diabetes.
In type 1 diabetes, the beta cells of the pancreas produce little or
no insulin, the hormone that allows glucose to enter body cells.
Once glucose enters a cell, it is used as fuel.
Without adequate insulin, glucose builds up in the bloodstream
instead of going into the cells. The body is unable to use this
glucose for energy despite high levels in the bloodstream,
leading to increased hunger.
In addition, the high levels of glucose in the blood causes the
patient to urinate more, which in turn causes excessive thirst.
Within 5 to 10 years after diagnosis, the insulin-producing beta
cells of the pancreas are completely destroyed, and no more
insulin is produced.
1.
2.
3.
4.
5.
6.
Symptoms
Increased thirst
Increased urination
Weight loss despite increased appetite
Nausea
Vomiting
Abdominal pain
Fatigue
Absence of menstruation
Signs and tests The following tests can be used to diagnose
diabetes:
1. Urinalysis shows glucose and ketone bodies in the urine,
but a blood test is required for diagnosis
2. Fasting blood glucose is 126 mg/dL or higher
3. Random (nonfasting) blood glucose exceeds 200 mg/dL
(this must be confirmed with a fasting test)
4. Insulin test (low or undetectable level of insulin)
5. C-peptide test (low or undetectable level of the protein Cpeptide, a by-product of insulin production)
The long-term goals of treatment are to prolong
life, reduce symptoms, and prevent diabetesrelated complications such as blindness, kidney
failure, and amputation of limbs.
These goals are accomplished through
education, insulin use, meal planning and weight
control, exercise, foot care, and careful selftesting of blood glucose levels.
Ovary
Ovaries are egg-producing
reproductive organs found in
female organisms. They are
part of the vertebrate female
reproductive system. Ovaries
in females are homologous
to testes in males. The term
gonads refers to the ovaries
in females and testes in
males.
Estrogen and progesterone are the most important in mammals.
These hormones serve many functions:
1. They induce and maintain the physical changes of puberty and
the secondary sex characteristics.
2. They support maturation of the uterine endometrium in
preparation of implantation of a fertilized egg.
3. They provide signals to the hypothalamus and pituitary that help
maintain the menstrual cycle.
4. Estrogen plays an important role in maintaining subcutaneous
fat, bone strength, and some aspects of brain function.
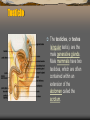
Testicle
The testicles, or testes
(singular testis), are the
male generative glands.
Male mammals have two
testicles, which are often
contained within an
extension of the
abdomen called the
scrotum.
1.
2.
1.
2.
Like the ovaries (to which they are homologous), testicles are
components of both the reproductive system (being gonads)
and the endocrine system (being endocrine glands). The
respective functions of the testicles are:
producing sperm (spermatozoa)
producing male sex hormones, of which testosterone is the
best-known
Both functions of the testicle, sperm-forming and endocrine, are
under control of gonadotropic hormones produced by the
anterior pituitary:
luteinizing hormone (LH)
follicle-stimulating hormone (FSH)
Cryptorchidism
Cryptorchidism is a medical term referring to absence
from the scrotum of one or both testes. This usually
represents failure of the testis to move, to "descend,"
during fetal development from an abdominal position,
through the inguinal canal, into the ipsilateral scrotum.
About 3% of full-term and 30% of premature infant boys
are born with at least one undescended testis, making
cryptorchidism the most common birth defect of male
genitalia. However, most testes descend by the first
year of life (the majority within three months), making
the true incidence of cryptorchidism around 1% overall.
1.
2.
3.
4.
5.
A testis absent from the normal scrotal position can be:
found anywhere along the "path of descent" from high in the posterior
(retroperitoneal) abdomen, just below the kidney, to the inguinal ring;
found in the inguinal canal;
ectopic, that is, found to have "wandered" from that path, usually outside the
inguinal canal and sometimes even under the skin of the thigh, the perineum,
the opposite scrotum, and femoral canal;
found to be undeveloped (hypoplastic) or severely abnormal (dysgenetic);
found to have vanished (also see Anorchia).
About two thirds of cases without other abnormalities are unilateral; 1/3
involve both testes. In 90% of cases an undescended testis can be palpated
(felt) in the inguinal canal; in a minority the testis or testes are in the abdomen
or nonexistent (truly "hidden").
Thyroid
The thyroid (from the Greek word for
"shield", after its shape) is one of the
larger endocrine glands in the body. It
is a double-lobed structure located in
the neck and produces hormones,
principally thyroxine (T4) and
triiodothyronine (T3), that regulate the
rate of metabolism and affect the
growth and rate of function of many
other systems in the body. The
hormone calcitonin is also produced
and controls calcium blood levels.
Iodine is necessary for the production
of both hormones. Hyperthyroidism
(overactive thyroid) and hypothyroidism
(underactive thyroid) are the most
common problems of the thyroid gland.
Physiologic effects of thyroid hormone
Regulates metabolic rate of all cells; protein, fat, and
carbohydrate catabolism; and nitrogen excretion
Regulates body heat production and heat-dissipating
mechanisms
Regulates protein synthesis and catabolism, ammo acid
incorporation into protein, and transcription of
messenger RNA
Increases gluconeogenesis and peripheral utilization of
glucose
Physiologic effects of thyroid hormone
Maintains appetite and secretion of gastrointestinal
substances
Maintains calcium mobilization
Stimulates
cholesterol synthesis and hepatic
mechanisms that remove cholesterol from the
circulation; stimulates lipid turnover and free fatty acid
release
Regulates hepatic conversion of carotene to vitamin A
Maintains
growth hormone secretion, skeletal
maturation, and tissue differentiation
Physiologic effects of thyroid hormone
Is necessary for muscle tone and vigor and normal skin
constituents
Maintains cardiac rate, force, and output
Affects respiratory rate, depth of oxygen utilization, and
carbon dioxide formation
Affects central nervous system development and
cerebration during first 2 to 3 years
Affects milk production during lactation and menstrual
cycle fertility
Maintains sensitivity to insulin and insulin degradation
Physiologic effects of thyroid hormone
Affects red cell production
Affects cortisol secretion, probably caused by
direct effect on adrenal glands and by increasing
ACTH secretion
T3 and T4 production and action
Thyroxine is synthesised by the follicular cells from free tyrosine
and on the tyrosine residues of the protein called thyroglobulin
(TG). Iodine, captured with the "iodine trap" by the hydrogen
peroxide generated by the enzyme thyroid peroxidase (TPO) and
linked to the 3' and 5' sites of the benzene ring of the tyrosine
residues on TG, and on free tyrosine. Upon stimulation by TSH
(see below), the follicular cells reabsorb TG and proteolytically
cleave the iodinated tyrosines from TG, forming T4 and T3 (in T3,
one iodine is absent compared to T4), and releasing them into
the blood. Deiodinase enzymes convert T4 to T3. Thyroid
hormone that is secreted from the gland is about 90% T4 and
about 10% T3.
Cells of the brain are a major target for thyroid hormone. Thyroid hormones
play a particularly crucial role in brain development during pregnancy]. A
transport protein (OATP1C1) has been identified that seems to be important
for T4 transport across the blood brain barrier. A second transport protein
(MCT8) is important for T3 transport across brain cell membranes.
In the blood, T4 and T3 are partially bound to thyroxine-binding globulin,
transthyretin and albumin. Only a very small fraction of the circulating hormone
is free (unbound) - T4 0.03% and T3 0.3%. Only the free fraction has hormonal
activity. As with the steroid hormones and retinoic acid, thyroid hormones
cross the cell membrane and bind to intracellular receptors (α1, α2, β1 and
β2), which act alone, in pairs or together with the retinoid X-receptor as
transcription factors to modulate DNA transcription.
Calcitonin
An additional hormone produced by the thyroid
contributes to the regulation of blood calcium levels.
Parafollicular cells produce calcitonin in response to
hypercalcemia. Calcitonin stimulates movement of
calcium into bone, in opposition to the effects of
parathyroid hormone. However calcitonin seems far
less essential than PTH, as calcium metabolism
remains clinically normal after removal of the thyroid,
but not the parathyroids.
The significance of iodine
In areas of the world where iodine (essential for the production of
thyroxine, which contains four iodine atoms) is lacking in the diet,
the thyroid gland can be considerably enlarged, resulting in the
swollen necks of endemic goitre.
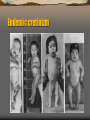
In humans, children born with thyroid hormone deficiency will
have physical growth and development problems, and brain
development can also be severely impaired, in the condition
referred to as cretinism. Newborn children in many developed
countries are now routinely tested for thyroid hormone deficiency
as part of newborn screening by analysis of a drop of blood.
Children with thyroid hormone deficiency are treated by
supplementation with synthetic thyroxine, which enables them to
grow and develop normally.
Endemic cretinizm
Diseases of the thyroid gland
Hyper- and hypofunction (affects about 2% of the population):
Hypothyroidism
(underactivity)
Hashimoto's thyroiditis /
thyroiditis
Ord's thyroiditis
Postoperative hypothyroidism
Postpartum thyroiditis
Silent thyroiditis
Acute thyroiditis
Iatrogenic hypothyroidism
Hyperthyroidism
(overactivity)
Thyroid storm
Graves-Basedow disease
Toxic thyroid nodule
Toxic nodular struma
(Plummer's disease)
Hashitoxicosis
Iatrogenic hyperthyroidism
De Quervain thyroiditis
(inflammation starting as
hyperthyroidism, can end as
hypothyroidism)
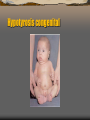
Hypotyrosis congenital
Hypotyrosis congenital
Thyroid hormone deficiency.
Thyroid hormone deficiency is always associated with
poor growth and delayed bone maturation.
Hypothyroidism that is present from birth causes severe
stunting of linear growth, which is evident early in life.
When the deficiency begins before the skeletal age of 9
or 10 years, the child maintains infantile proportions
with short legs compared to the length of the spine; he
tends to be pale, sluggish, inactive, and obese; and
intellectual achievement at school deteriorates.
Acquired
hypothyroidism varies with the degree
and duration of the deficiency, but skeletal age is
delayed if the condition has been present more
than 12 months .
Parathyroid Glands
1. Parathyroid glands are embedded in the thyroid
glands.
2. Parathyroid hormone (PTH) increases blood calcium
levels.
-PTH stimulates osteoclasts and inhibts osteoblasts.
-PTH promotes calcium reabsorption by the kidneys and
the formation of active vitamin D by the kidneys.
Active vitamin D increases calcium absorption by the
intestine.
3. A decrease in blood calcium levels stimulates PTH
secretion.
Growth hormone deficiency.
Growth hormone deficiency, associated with
hypopituitarism, inhibits somatic growth in all cells of the
body. Although children with hypopituitarism are normal
at birth, they show growth patterns that progressively
deviate from the normal growth rate, often beginning in
infancy. The chief complaint in most instances is short
stature. Of those who seek help, boys outnumber girls
three to one. Skeletal proportions are normal for the
age, but these children appear younger than their
chronologic age, tend to be
relatively inactive, and are less apt to participate in
aggressive, sporting-type activities. Bone age is nearly
always retarded but is closely related to height age; the
degree of retardation depends on the duration and
extent of the hormonal deficiency. Diminished function
of recent onset may show little retardation in skeletal
age, whereas children with a long-standing deficiency
may evidence a skeletal age only 40% to 50% of their
chronologic age.
In children with a partial growth hormone deficiency, the
growth retardation is less marked than in children with a
growth hormone deficiency.
Growth hormone deficiency may be attributed to an
idiopathic or organic etiology. The extent of idiopathic
growth hormone deficiency may be complete or partial,
but the cause is unknown. It is frequently associated
with other pituitary hormone deficiences, such as
deficiences of thyroid-stimulating hormone and ACTH;
Thus
it is theorized that the disorder is probably
secondary to hypothalamic deficiency. It has also
been observed that there is a higher than
average frequency in some families, which
indicates a possible genetic etiology in a number
of instances.
Sex hormone deficiency.
Sex hormone deficiency that causes delayed puberty
can occur as a result either of pituitary dysfunction or of
hypogonadism. A hypofunctioning pituitary gland, as
briefly discussed in the preceding segment on
endocrine dysfunction, can produce a deficiency in
either the gonadotropic hormones, which retards
maturation of the gonads, or growth hormone, which will
diminish total growth during childhood.
Cortisol excess.
Cortisol excess as a result of organic causes or of
prolonged cortisone therapy also has an adverse effect
on growth in children. This effect is produced by direct
action on growing cartilage, interference with production
of growth hormone, or interference with the response to
or production of somatomedin. Because of the growthsuppressing effect of cortisone in excess of minimal
requirements, therapy is limited to short-term
administration whenever possible.
Syndromes of primary gonadal
failure.
The most frequently seen disorders associated with
primary gonadal failure are the sex chromosomal
defects categorized collectively as gonadal dysgenesis,
principally
Turner's
syndrome.
Chromosomal
impairment of male sexual function is most commonly
caused by Klinefelter's syndrome. Derangements that
become apparent at puberty are more common. Clinical
presentation in the female may be masculinization,
sexual infantilism or hypoplasia, primary absence of
menstruation (amenorrhea),
or
abnormally scanty or infrequent menstruation
(oligomenorrhea or hypomenorrhea).
The child with an endocrine dysfunction
The major chemical regulators of the body are
the internal secretions and their secreting cells,.
The function of the endocrine system is to secrete
intracellularly synthesized hormones into the circulation
where they are transported to nearby or distant sites to
stimulate, catalyze, or serve as pacemaker substances
for metabolic processes.
Together with the closely related but more rapidly
reacting nervous system, they serve to integrate the
various physiologic functions of the organism in
adjusting to external and internal environmental
demands.
Endocrine
substances even in extremely small
concentrations are effective in modifying
metabolism, behavior, and development.
What kind of training do
pediatric endocrinologists have?
Pediatric endocrinologists are medical doctors who
have had
•Four years of medical school
• Three years of pediatric residency
• Three or more years of fellowship training in
pediatric endocrinology
What types of treatment do pediatric endocrinologists provide?
Pediatric endocrinologists diagnose, treat, and manage
hormonal disorders including the following:
•Growth problems, such as short stature
• Early or delayed puberty
• Enlarged thyroid gland (goiter)
•Underactive or overactive thyroid gland
• Pituitary gland hypo/hyper function
•Adrenal gland hypo/hyper function
• Ambiguous genitals/intersex
•Ovarian and testicular dysfunction
• Diabetes
•Low blood sugar (hypoglycemia)
• Obesity
•Problems with Vitamin D (rickets, hypocalcemia)
Pediatric endocrinologists—the
best care for children
Children are not just small adults. As growing individuals they have
special needs related to growth and development. In addition,
their psychological needs are different from those of adults.
Hormone problems affecting growth or sexual development can
have significant effects on a child’s physical and emotional wellbeing. Pediatric endocrinologists are sensitive to these issues.
A pediatric endocrinologist cares for your child in a setting that is
appropriate for children and teens. Support personnel, including
nurses, psychologists, pediatric diabetes educators, and
nutritionists, are all attuned to the needs of children and teens.