* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Slide 1
Survey
Document related concepts
Transcript
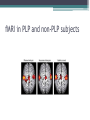
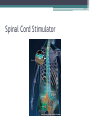
Post-Amputation Pain NSW Physiotherapists in Amputee Rehabilitation 14 June 2013 Dr R. Craig Davenport Rehabilitation Physician Post-Amputation Pain • Phantom Limb Pain • Residual Limb Pain (Stump Pain) • Phantom Sensations Residual Limb (Stump) Pain • More of an issue in immediate post-amputation period • Descriptors: ▫ ▫ ▫ ▫ ▫ Sharp Burning Electrical-like Skin-sensitive Localised to a superficial incision, or deep pain, or generalised in the residual limb • Residual limb pain incidence may be as high as 74% and can persist for many years Phantom Sensations • Defined as non-painful perceptions emanating from lost body part after deafferentation or amputation • Common ▫ ~1/3 in first 24 hours ▫ ¾ at 4 days ▫ 90% within 6 months • Doesn’t require amputation Can occur with Spinal Cord Injury and Brachial plexus avulsion • Usually in hands and feet (large cortical representation) • Not restricted to limbs, can occur in ear, teeth etc Phantom Sensations • Types of sensations: ▫ Kinetic – perceived movement of a body part ▫ Kinesthetic – size, shape, or position of body part ▫ Exteroceptive perceptions – touch, pressure, tingling, temperature, itch, vibration • Associated with Phantom Pain phantom pain rare in those without phantom sensations • Often experience telescoping over time Phantom Limb Pain • Defined as an unpleasant sensation in distribution of the lost or de-afferentated body part Phantom Limb Pain • First described by 16th century French military surgeon Ambrose Pare • The term “Phantom Limb Pain” coined by 19th century civil war surgeon Silas Weir Mitchell • Incidence of PLP common – between 40% and 80% • Similar between civilian and military, and between different aetiologies • Not only in amputations, but also occurs in congenital limb deficiency Risk Factors for Phantom Limb Pain • Presence of pre-amputation pain severity ▫ Relationship to pre-amputation pain severity exists for first few months, but probably not for pain >6 months post-op Fig 1 Pre‐amputation pain ≥20 increases the risk of phantom pain ≥20 after 1 week and 3 months (on a VAS, 0–100). Nikolajsen L , and S. Jensen T Br. J. Anaesth. 2001;87:107116 ©2001 by Oxford University Press Risk Factors for Phantom Limb Pain • Residual limb pain severity correlates to PLP severity • Upper Limb Amputations > Lower Limb Amputations • Females > Males • Time after amputation ▫ ▫ ▫ ▫ Onset of PLP has two peaks: within 1st month & a year after amputation Typically occurs within first 6 months Prevalence decreases over time Like Phantom Sensations, up to 2/3 experience Telescoping over time Phantom Limb Pain - Descriptors • Pain description can change over time ▫ exteroceptive-like (knife-like, stabbing) in proximal limb or more generalised ▫ proprioceptive-like (burning, squeezing) localised to distal areas of amputated limb PLP descriptors • Most common: ▫ ▫ ▫ ▫ Tingling Throbbing Piercing Pins and needles • Others: ▫ ▫ ▫ ▫ ▫ ▫ Sharp Shooting Electrical-like Dull Squeezing Cramping Factors influencing pain • Stress/anxiety/depression/emotional triggers contribute to persistence or exacerbation of PLP • Presence of depression associated with characterisation of more severe pain • Possible genetic predisposition to development of neuropathic pain Mechanisms of Phantom Pain • Peripheral • Central ▫ Spinal ▫ Supra-spinal (Brain & Brainstem) Mechanisms of PLP • Peripheral ▫ Tissue injury ▫ neural injury ▫ de-afferentation ▫ Proximal portion of nerve sprouts to form Neuroma upregulation of voltage-gated sodium channels, downregulation of K-channels. Development of ephapses (nonfunctional connections between neurons) hyper-excitability and increased spontaneous discharge ▫ Chemosensitivity to circulating catecholamines (eg adrenalin) pain increased with stress Spinal Cord – Dorsal Horn changes • Axonal sprouts form connections with neurons in receptive field • Loss of afferent input causes reduced descending inhibitory input from reticular formation in brainstem • Central sensitisation ▫ Expansion of neuronal receptive field, hyper-excitability ▫ Increased NMDA receptor activity – mediated by Substance P, tachykinins, neurokinins at dorsal horn ▫ “Wind-up” – up-regulation of receptors in the area ▫ Loss of target neurons for descending inhibitory pathways ▫ Spinal disinhibition due to loss of local inhibitory intersegmental spinal interneurons • But ▫ spinal ablative treatments (cordotomy tractotomy etc) often fail to give long term relief Brain Changes – The Homunculus Brain changes • Cortical re-organisation in primary somatosensory and motor cortex ▫ Extent of re-organisation related to degree of pain and size of deafferented region • Body-schema – Neuromatrix and Neurosignature – loss of input causes abnormal neurosignature to develop • Incongruence of motor intention and sensory feedback – involves parietal and frontal lobes involved fMRI in PLP and non-PLP subjects Psychogenic mechanisms • Not well supported by evidence • previously thought to be associated with passive coping styles and catastrophising behaviour traits Pain Limbic Connections What Causes might be identified and possibly treated? Residual Limb Pain - Causes • Post-surgical nocipceptive tissue trauma pain • Neurogenic causes- clinically significant neuroma • Prosthogenic causes ▫ Poor fitting socket Too tight/lack of distal contact, insufficient bony relief, Too loose, excessive end-bearing, pistoning ▫ Socket malalignment with torque forces in weight-bearing ▫ Incorrect donning of prosthesis ▫ Incorrect use of socks • Adherent Scar tissue • Heterotopic ossification – initially acute inflammatory pain and then pressure effects Residual Limb Pain - Causes • Arthrogenic – eg OA of knee • Ischaemic – ongoing poor vascular supply • Sympathetically-maintained – eg Complex Regional Pain Syndrome • Referred from spine eg Radicular/Facet Joint/SI Joint pain generator • Stump or wound Infection • Pain from associated injuries • Pain from co-morbidities • Musculoskeletal Pain from Gait abnormalities How to tell…… • Referred from facet joint/ SI joint/ radiculopathy ▫ diagnostic blocks may help • Ischaemia ▫ transcutaneous oxygen tension <20mmHg • Neuroma ▫ Palpation point neuropathic pain, Ultrasound/MRI • Prosthesis-related pain ▫ pain on weight bearing, or after prolonged wearing, skin or soft tissue changes ▫ may prompt socket adjustments which may relieve pain • Stump infections – soft-tissue or bone ▫ blood tests/ultrasound/MRI/Nuclear imaging studies • Bone spurs ▫ palpation and plain radiographs Treatment Treatment Approaches • Few controlled trials to guide treatment • Often extrapolation of treatments for neuropathic pain • Multidisciplinary and Multi-modal Approach most successful • Multimodal approach ▫ ▫ ▫ ▫ ▫ ▫ ▫ Injections Pharmacotherapies Physical therapies Psychological therapies Complimentary and alternative therapies Surgery Preventative Measures Pharmacologic therapies • Common oral medications: ▫ Simple Analgesics – Paracetamol & NSAIDs ▫ Opioids – some evidence for morphine and tramadol Effective for PLP, possibly reduces cortical re-organisation Better when combined with other agents ▫ Anti-depressants Tricyclic antidepressants/Serotonin Re-uptake inhibitors, Sodium channel blockade, NMDA antagonist effects - mixed results for PLP Mirtazepine Duloxetine ▫ Anti-convulsants Gabapentin – mixed results Carbamazepine Pregabalin (Lyrica) Pharmacologic therapies - uncommon • Calcitonin – mixed results, mechanism of effect unclear – not used • NMDA receptor antagonists – blocks cascade leading to sensitisation of Wide Dynamic Range neurons in spinal cord ▫ Ketamine – short follow-up periods, psychogenic side effects ▫ Dextromethorphan – not used routinely ▫ Memantine – mixed results in RCTs – not used • Others ▫ Sodium channel blockers – lignocaine/mexiletine – not effective ▫ B-blocker - Propranolol ▫ Ca-channel blocker - Nifedipine Injection Therapies • More useful for residual limb pain than phantom limb pain ▫ ? Due to greater contribution of peripheral mechanisms in RLP compared with PLP • Regional nerve blocks (anaesthetics/corticosteroids) – often not long-lasting • Botulinum toxin A perineural blocks – currently under study, not enough evidence yet • Pulsed Radiofrequency – small study • TNFα inhibitor (etanercept) perineural injection– small study • Sympathetic nerve blocks – small study • Ambulatory Continuous Peripheral nerve block for 6 days – small cross-over study – promising long term improvements in 2/3 subjects Prosthetic Approaches • Prosthetist review, adjustment of socket fit • Alignment changes • Replacement socket if stump volume change, weight change etc • Utilise different prosthetic componentry eg. silicon/urethane liners, shock absorbers, torsion adaptors, multi-axial ankles • Shift weight bearing away from stump: thigh-lacers, ischial bearing prostheses – much less common since Silicon available Non-Pharm Treatments • Psychological interventions – aim to facilitate adaptation to pain, body image, negative emotions • EBM limited for these approaches • Cognitive Behavioural Therapy – no RCTs; RCT combining CBT and mirror therapy in progress • Hypnosis – small RCT showed benefit, 3 sessions • Guided imagery – anecdotal • Biofeedback – anecdotal • Relaxation Techniques Mirror Therapy Mirror neurons in the brain – fire when perform movement or when observe movement Activation may modulate somatosensory inputs and block protopathic pain perception in phantom limb i.e. lessens effect of deafferentation RCTs one +ve, one –ve Recent study added illusory touch stimulation for cases where movement caused increased pain (Schmalzl et al 2013) Mirror therapy – influencing cortical re-organisation Reduction of PLP with fMRI evidence of reduction in re-organisation Non-Pharm Treatments • Acupuncture – No RCTs, only descriptive studies • TENS – shown to be helpful ▫ ?Contralateral limb ▫ Low-Freq, hi intensity • Laser • Ultrasound/Heat – musculoskeletal pain Non-Pharm Treatments • Successful rehabilitation may reduce the amount of pain • Stump massage and familiarisation/desensitisation • Early prosthetic use ▫ In upper extremity amputees, phantom pain was decreased by the use of a prosthesis which allowed extensive use of the affected limb but not with cosmetic prosthesis use • Oedema control/Use of stump shrinker/RRD Early weight-bearing?? • No consensus on the impact of weight bearing on early wound healing • Possible benefits for reducing oedema and stimulation of circulation, reducing cortical reorganistaion • Possible detrimental effects of excessive loading damaging fragile new tissue and harming the healing response • Still no standard protocols for the amount, time, and advancement of weight bearing • Published studies use many different weight-bearing protocols • Main concern will be for poorly vascularised limbs or friable skin • My approach… anytime after 1 week if the tissues look good, but logistics mean that rarely less than 2-3 weeks post-op, otherwise after clips out and suture line healed in questionable stumps Surgical Intervention • Stump Revision occasionally useful for difficult to fit stumps • Clinically significant neuromas may require resection if prosthetic changes not sufficient mixed results with excision • Excision of Heterotopic bone and bone spurs – may recur • Neurosurgical procedures: ▫ ▫ ▫ ▫ DREZ lesioning Spinal Cord Stimulation – evidence for PAP less robust than for other neuropathic pain states Peripheral Nerve Stimulation – might work if one or two nerve territories affected, may not work if spinal or cortical re-organisation Deep Brain Stimulation – PVG, thalamic nuclei, motor cortex – case series evidence – mixed results for PAP • Electroconvulsive therapy ▫ A case report Spinal Cord Stimulator Surgical Intervention • Stump Revision occasionally useful for difficult to fit stumps • Clinically significant neuromas may require resection if prosthetic changes not sufficient mixed results with excision • Excision of Heterotopic bone and bone spurs – may recur • Neurosurgical procedures: ▫ ▫ ▫ ▫ DREZ lesioning Spinal Cord Stimulation – evidence for PAP less robust than for other neuropathic pain states Peripheral Nerve Stimulation – might work if one or two nerve territories affected, may not work if spinal or cortical re-organisation Deep Brain Stimulation – PVG, thalamic nuclei, motor cortex – case series evidence – mixed results for PAP • Electroconvulsive therapy ▫ A case report Deep Brain Stimulation Surgical Intervention • Stump Revision occasionally useful for difficult to fit stumps • Clinically significant neuromas may require resection if prosthetic changes not sufficient mixed results with excision • Excision of Heterotopic bone and bone spurs – may recur • Neurosurgical procedures: ▫ ▫ ▫ ▫ DREZ lesioning Spinal Cord Stimulation – evidence for PAP less robust than for other neuropathic pain states Peripheral Nerve Stimulation – might work if one or two nerve territories affected, may not work if spinal or cortical re-organisation Deep Brain Stimulation – PVG, thalamic nuclei, motor cortex – case series evidence – mixed results for PAP • Electroconvulsive therapy ▫ A case report Preventative Treatments • Pre-emptive analgesia/anaesthesia ▫ Pre-op, intra-op, early post-op period (<2 weeks) – goal to avoid long term spinal sensitisation • Still no definitive evidence • Prevention of Acute PLP ▫ Epidural treatments – 3 trials – mixed results, best quality study no benefit ▫ Regional nerve blocks – 3 studies – no effect ▫ IV calcitonin – 1 study – no benefit ▫ TENS – 1 study, no benefit • Prevention of chronic PLP ▫ Pre-op epidural anaesthesia – studies mixed but may suggest need to be started at least 24 hours pre-op May not need epidural Rx, other forms of good pre-op pain control may be equivalent ▫ Perineural anaesthesia – mixed results ▫ Prophylactic gabapentin from D1-30 not shown to prevent PLP ▫ TENS – RCT showed lower incidence at 4 months but no benefit at 4 weeks and 1 year, but improved wound healing time and lower re-amputation rate ▫ Mirror therapy prophylactically – small case series showed promise Cochrane Review 2012 – Pharmacologic Interventions for PLP • • • • • • Morphine - Short-term effect for pain relief Ketamine – analgesic effects Gabapentin – trend towards pain relief Amitriptyline – not effective for PLP Memantine – not effective Calcitonin – variable findings • i.e. NOT MUCH HELP Thankyou