* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download The perception of pain
Survey
Document related concepts
Transcript
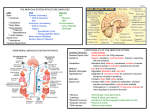
The perception of pain Ching-Liang Lu What is pain? • Pain is an unpleasant sensory and emotional experience associated with actual or potential tissue damage, or described in terms of such damage IASP – International Association for the Study of Pain 2009 What is pain? • Pain is – subjective • not a stimulus, but an experience – protective (acute) – modified by developmental, behavioural, personality and cultural factors • Associated signs are crying, sweating, increased heart rate, blood pressure, behavioural changes etc Pain • Dimensions of the ‘PAIN” – Sensory-discriminative – Affective-motivate – Cognitive Nociceptive vs Neuropathic Pain Nociceptive Pain Caused by activity in response to potentially tissue-damaging stimuli Neuropathic Pain Initiated or caused by primary lesion or dysfunction in the nervous system Phantom pain Postherpetic Arthritisneuralgia Pos postoperative pain Sickle cell toperative Mechanical crisis lowpain arthritis, back pain Neuropathic low back pain Sports/e sports or exercise injuries xercise injuries *Complex regional pain syndrome Trigeminal neuralgia Central poststroke pain Distal polyneuropathy (eg, diabetic, HIV) Nociceptive pain: Tissue injury/inflammation Postherpetic neuralgia Acute vs Chronic Pain Characteristic Acute Pain Chronic Pain Cause Generally known Often unknown Duration of pain Short, well-characterized Persists after healing, 3 months Treatment approach Resolution of underlying cause, usually self-limited Underlying cause and pain disorder; outcome is often pain control, not cure What is Acute Pain? • • • • • • Physiologic response to tissue damage Warning signals damage/danger Helps locate problem source Has biologic value as a symptom Responds to traditional medical model Life temporarily disrupted (self limiting) Acute pain is not that bad. What is Chronic Pain? • Chronic pain is persistent or recurrent pain, lasting beyond the usual course of acute illness or injury, or more than 3 - 6 months, and adversely affecting the patient’s wellbeing • Pain that continues when it should not Domains of Chronic Pain Quality of Life Physical functioning Ability to perform activities of daily living Work Recreation Social Consequences • Marital/family relations • Intimacy/sexual activity • Social isolation Psychological Morbidity Depression Anxiety, anger Sleep disturbances Loss of self-esteem Socioeconomic Consequences • Healthcare costs • Disability • Lost workdays Pain measurement Nociceptors • 3 classes of nociceptors –Mechanical: pinch, punctate, squeeze –Thermal: above 45C or below 5C –Polymodal: mechanical, thermal, chemical Somatic receptor types Thermosensation Heat DRG • 45% small- to medium-diameter neurons: threshold of ∼42°C heat-sensitive C and type II Aδ fibres TRPV-1 receptor • 5-10% medium- to large-diameter cells: threshold of ∼51°C type I Aδ TRPV-2 receptor • TRPV-3 TRPV -4: warm range 31∼39°C Thermosensation Cold •TPRVM8 receptor: ∼26°C ; (cool to cold) express in 10-20 % of small-diameter neuron • TRPA1: ∼17°C painful cold ? TRP channel family Mechanosensation Mechanoreceptor: •? Yet to be identified in mammalian • non-mammalian • Bacteria: MscL, MscS, DEG/EnaC ion channel, • Yeast, flies, worms: TRP channel • Fly, Zebra fish: NOMPC (TRP channel) AFFERENT (SENSORY) NEURON Nociceptive afferents First pain: Sharp, faster A-delta fibers Compound Action Potential Second pain: Dull, slower C-fibers Blocking each nerve blocks the sensation SPINAL CORD LAMINAE LAMINA PREDOMINANT FUNCTION INPUT NAME I SOMATIC NOCICEPTION THERMORECEPTION Aδ, C MARGINAL LAYER II SOMATIC NOCICEPTION THERMORECEPTION C, Aδ SUBSTANTIA GELATINOSA III SOMATIC NOCICEPTION MECHANORECEPTION Aβ, Aδ NUCLEUS PROPRIUS IV MECHANORECEPTION Aβ, Aδ, NUCLEUS PROPRIUS V VISCERAL & SOMATIC NOCICEPTION & MECHANORECEPTION Aβ, Aδ, C NUCLEUS PROPRIUS WDR NEURONS VI MECHANORECEPTION Aβ NUCLEUS PROPRIUS VII SYMPATHETIC VIII IX X MOTOR INTERMEDIOLATERAL COLUMNS Aβ MOTOR HORN Aβ MOTOR HORN Aβ CENTRAL CANAL Dorsal horn Lamina of spinal cord Referred pain • Signals from muscles and viscera can be felt as pain elsewhere • Example: myocardial infarction and angina can be felt in chest and left arm • Mechanism: convergence of afferents muscle/ viscera afferents and somatic afferents. • Convergence on the same projection neurons in the dorsal horn • The brain cannot tell the difference Neurotransmitters • Fast synaptic potentials – Glutamate (amino acid, excitatory) – Efficient reuptake of amino acids – Range: postsynaptic neurons in vicinity • Slow synaptic potentials – Neuropeptides e.g. Substance P(excitatory), GABA (inhibitory) – No reuptake mechanisms – Range: diffusion, many neurons, unlocalized nature of pain • Other Neuropeptides – Released and increased in persistent pain conditions – Enhances and prolong the actions of glutamate Substance P in dorsal horn Pain modulation below spinal level 1. PERIPHERAL 2. CENTRAL 1. Peripheral (Sensitization) TISSUE INJURY RELEASE OF SUBBSTANCE-P AND GLUTAMATE CHEMICAL MEDIATORS OF INFLAMMATION STIMULATE NOCICEPTORS IN THE PERIPHERY Peripheral Sensitization NSAID Peripheral sensitization 53C 30 sec at site A&D Central sensitization for chronic pain: wind-up • “Wind-up” – Response to second stimulus is stronger than the response to the first one in C fiber • Change in temporal integration "Wind-up" is NMDA mediated. Response with and without an NMDA antagonist. Control NMDA antagonist 40 30 20 10 0 0 10 Stimulus number 20 Spinal microglia activation in the chronic visceral pain (chronic pancreatitis) Liu PY, Lu CL. Gastroenterology 2012; 142: 165-73. Spinal microglia in visceral pain TNBS I.T. tetracycline (microglia inhibitor) Liu PY, Lu CL. Gastroenterology 2012; 142: 165-73. Ascending pathways Spinal cord ThalamusCortex Ascending pathways Spinal cord ThalamusCortex • Thalamic nuclei – Lateral nuclear group: spinothalamic tract, NS and WDR, laminae I and V, small receptive fields, encoding location of injury – Infarction Thalamic syndrome (spontaneous burning pain, allodynia) – Medial nuclear group: spinoreticulothalamic tract, laminae VII and VIII New pathway for visceral pain: Dorsal column pathway Lateral system: S1/2 Discrimitive component Thalamus Medial system: Affective component Neuroimaging of acute pain Cutaneous pain Muscle pain Visceral pain PFC PFC ACC PFC ACC PCC PPC PPC Insula PCC PCC Chen et al. Tooth pain Insula Thalamus Insula Vermis Amygdala Vermis Lin et al. (preliminary) Lu et al., 2004 Niddam et al., 2002 Somatosensory area Cortical neuronal correlates upon balloon distension in GI tract (volunteers) Esophagus Rectum Antrum Fundus Mertz H. Gastroenerology 2000; 118:8342 Ladabaum U. Gastroenerology 2001; 120:369 Aziz Q. Gastroenerology 1997; 113:50 Lu CL Neurogastroenterol 2004;16:575 Gastroenerology 2005; 128:1529 Brain Pain perception No Brain, No Pain Rene Descartes (1596-1650) Pain modulation (Central) • Gate control theory • Descending inhibition Gate control theory • P.D. Wall & Melzack (1965) • “There is an interaction between pain fibres and touch fibre input at the spinal cord level in the form of a ‘gating mechanism’ Gate control theory pain is felt + pain gate is opened When pain fibre is stimulated, gate will be opened & pain is felt Gate control theory pain is not felt touch + pain gate is closed When pain and touch fibres are stimulated together, gate will be closed & pain is not felt Gate control theory • This theory provided basis for various methods of pain relief – Massaging a painful area – Applying irritable substances to a painful area (counter-irritation) – Transcutaneous Electrical Nerve Stimulation (TENS) – Acupuncture ? Descending inhibition • Direct stimulation of PAG produces analgesia – Inhibits firing of nociceptive neurons in lamina I and V – Descending pathway recruited: PAG excites rostroventral medulla/ nucleus raphe magnus (5HT); LC(NE); VTA (DA) • Role of Opioid receptor – Opioid-induced analgesia via endogenous opioid receptors (-, δ-, κ-) in descending pathway ( PAG, ventral medulla, superficial dorsal horn) – Endogenous opioid peptide :enkephalins, βendorphin, dynorphin – Stress-Induced Analgesia (SIA) “Pain is a more terrible lord of mankind than even death itself” “疼痛比死亡更可怕” Dr. Albert Schweitzer (1875-1965)