* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download pptx - Yale University
Magnetic circular dichroism wikipedia , lookup
Heat transfer physics wikipedia , lookup
Photoelectric effect wikipedia , lookup
Electron configuration wikipedia , lookup
Ultraviolet–visible spectroscopy wikipedia , lookup
Ultrafast laser spectroscopy wikipedia , lookup
Astronomical spectroscopy wikipedia , lookup
Metastable inner-shell molecular state wikipedia , lookup
Cross section (physics) wikipedia , lookup
Reflection high-energy electron diffraction wikipedia , lookup
Vibrational analysis with scanning probe microscopy wikipedia , lookup
Atomic theory wikipedia , lookup
Chemical bond wikipedia , lookup
Rutherford backscattering spectrometry wikipedia , lookup
Biological small-angle scattering wikipedia , lookup
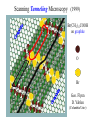
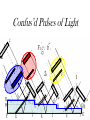
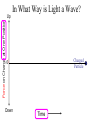
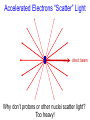
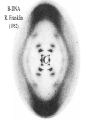
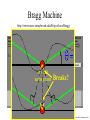
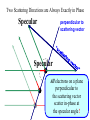
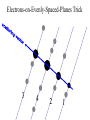
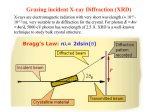
Chemistry 125: Lecture 5 Sept. 10, 2010 X-Ray Diffraction In the last 25 years various manifestations of Scanning Probe Microscopy, such as AFM, STM, and SNOM, have enabled chemists to “feel” individual molecules and atoms. SPM techniques are not quite good enough yet to study how electrons are distributed in bonds. Because light is scattered predominantly by the charged particles with the smallest mass, the electron distribution in molecules can be determined by x-ray diffraction. The roles of molecular pattern and crystal lattice repetition can be illustrated by shining a visible laser through diffraction masks to generate scattering patterns reminiscent of those encountered in X-ray studies of ordered solids. For copyright notice see final page of this file Despite Earnshaw, might there still be shared-pair bonds and lone pairs? Only one of these five cantilevers is used Atomic Gold in any one experiment. They differ in Force coated stiffness and ability to twist. Microscopy Hair Si Chip 1.4 mm The smallest scale division is 20 mm Scanning Electron Micrographs of AFM Cantilever Tip Radius of curvature ~20 nm 1mm ©The IN-VSEE Project; Arizona State University 1999 http://invsee.asu.edu/Invsee/listmod.htm Tips Cantilever/Chip Holder Cantilever/Chip Holder Sensitive to < 1 molecule change in height ! AFM traces at 1 min intervals of part of the surface of a benzoin crystal dissolving in 95% water / 5% n-propanol. The larger pit is 5.1 nm deep. Each ledge is one “unit cell” (1.7 nm) high. fast Ledge Dissolution Rate slower slow fast 5 mm (~600 molecules) Scanning Tunneling Microscopy (1999) Br(CH2)11COOH on graphite O Br Geo. Flynn D. Yablon (Columbia Univ) Quantum corral Permission limited by IBM Click to link to copyright webpage http://www.almaden.ibm.com:80/vis/stm/corral.html STM Image of Fe atoms on Cu (1993) Reprint Courtesy of International Business Machines Corporation, copyright 1993 © International Business Machines Corporation High Resolution Requires Small Probe No Barriers Horizontal Line Close Barriers One Broad Hump Distant Barriers Distorted Humps Rolling Wheel with Chalk on Axle to Trace Chalk-Trough Profile Pentacene on Cu Scanned with a Single-Atom Tip at 5K 10 v/m! 10 2.5v Cu L. Gross, et al., Science, Aug. 28, 2009 SNOM Light Scanning Near-Field Optical Microscope Glass Fiber Aluminum Coating 100 nm Aperture Sample (scanned) Emitted Light Lens Detector mm scale W. Brocklesby www.orc.soton.ac.uk by permission red wavelength SNOM image of nanofabricated material Scanning Probe Microscopies (AFM, STM, SNOM) are really powerful. Sharp points can resolve individual molecules and even atoms but not bonds Lux A lonely architectural curiosity on Sterling Chemistry Laboratory at Yale University (1923) Micrographia Robert Hooke (1665) “But Nature is not to be limited by our narrow comprehension; future improvements of glasses may yet further enlighten our understanding, and ocular inspection may demonstrate that which as yet we may think too extravagant either to feign or suppose.” Strong 400 nm Scattering No 800 nm Scattering “Thickness” ~ 200 nm Oil Path Difference = 400 nm =1l = 0.5 l Interference upon Scattering Water Hooke: Observ. IX. Confus’d Pulses of Light Of the Colours observable in Muscovy Glass, and other thin Bodies. Chris Incarvito’s New Toys User Operated - CCD Detector ~$200K X-Ray Tube ~$350K Image Plate "Seeing" Individual Molecules, Atoms, and Bonds? Problem: l What IS light? In What Way is Light a Wave? Force on Charge at One Position Up Charged Particle 0 Down Time Force at Different Positions - OneTime In What Way is Light a Wave? Up Charged Particle 0 Down Position Accelerated Electrons “Scatter” Light direct beam Why don’t protons or other nuclei scatter light? Too heavy! Interference of Ripples Angular Intensity Distribution at great distance depends on Scatterer Distribution at the origin By refocussing, a lens can reassemble the information from the scattered wave into an image of the scatterers. But a lens for x-rays is hard to come by. Be sure to read the webpage on x-ray diffraction. "Seeing" Molecules, Atoms, Bonds Collectively by X-Ray Crystallography SPM “feels” them Individually Blurring Problem from Motion and Defects Blurring Problem Time Averaging Space Averaging in Diffraction (Cooperative Scattering) Advantage for SPM (Operates in Real Space) Shadow of Frau Röentgen’s hand (1896) In 1895 Röntgen Discovers X-Rays In 1912 Laue Invents X-Ray Diffraction CuSO4 Diffraction (1912) Wm. Lawrence Bragg (1890-1971) Determined structure of ZnS from Laue's X-ray diffraction pattern (1912) (1915) Courtesy Dr. Stephen Bragg Youngest Nobel Laureate B-DNA R. Franklin (1952) Science, 11 August 2000 >100,000 atoms + hydrogens! 25 nm (250 Å) What can X-ray diffraction show? Molecules? Atoms? Bonds? How does diffraction work? Like all light, X-rays are waves. Wave Machines Bragg Machine http://www.eserc.stonybrook.edu/ProjectJava/Bragg/ in & out same phase Breaks? by permission, Konstantin Lukin Two Scattering Directions are Always Exactly in Phase Specular perpendicular to scattering vector Specular All electrons on a plane perpendicular to Direct the scattering vector scatter in-phase at the specular angle ! Electrons-on-Evenly-Spaced-Planes Trick 3 4 2 1 Electrons-on-Evenly-Spaced-Planes Trick Total Electrons Suppose l & angle such that: 3 +4 1l +2 2l 3l 3 4 2 1 +1 10 Net in-phase scattering Electrons-on-Evenly-Spaced-Planes Trick Total Electrons 3 3 +4 -4 0.5l +2 +2 1l 1.5l 3 4 2 1 Suppose first path difference is half a wavelength, because of change in l (or angle) +1 -1 10 0 Net in-phase scattering spot spacing = 10.8 cm ………………….. Q. What is the line spacing? DIFFRACTION MASK (courtesy T. R. Welberry, Canberra) 10.6 m View from Ceiling 633 nm To see and understand these diffraction images, study the course X-ray website: https://webspace.yale.edu/chem125/125/xray/diffract.html and particularly the section: https://webspace.yale.edu/chem125/125/xray/laserdiffraction.htm End of Lecture 5 Sept 11, 2009 Copyright © J. M. McBride 2009. Some rights reserved. Except for cited third-party materials, and those used by visiting speakers, all content is licensed under a Creative Commons License (Attribution-NonCommercial-ShareAlike 3.0). Use of this content constitutes your acceptance of the noted license and the terms and conditions of use. Materials from Wikimedia Commons are denoted by the symbol . Third party materials may be subject to additional intellectual property notices, information, or restrictions. The following attribution may be used when reusing material that is not identified as third-party content: J. M. McBride, Chem 125. License: Creative Commons BY-NC-SA 3.0