* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Atoms, Elements, and Compounds
Survey
Document related concepts
Transcript
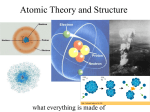
Atoms, Elements, and Compounds Why do we study Chemistry in Earth Science? Chemistry is the study of matter. Matter is anything that has mass and takes up space. All living and non living things we study in Earth Science are made up of matter! ATOMS Atoms are the building blocks of matter! Atoms are very tiny but are made up of even smaller particles called neutrons, protons, and electrons. Neutrons and Protons Neutrons and protons are located in the center of an atom, called the nucleus. Protons are positively charged particles. Neutrons are particles that have no charge. Electrons Electrons are negatively charged particles located outside the nucleus. Electrons are constantly moving around an atom’s nucleus in energy levels. There are an equal number of protons and electrons in an atom, so the overall charge is zero How do I tell how many protons, neutrons, and electrons are in an atom?? 1. Look at your Periodic Table of Elements. 2. The atomic number is the number of protons in an atom of an element. Atoms must have equal numbers of protons and electrons…so, in our atom of krypton, we must have 36 electrons since it contains 36 protons. 3. And the number of neutrons is… The atomic weight is basically a measurement of the total number of particles in an atom’s nucleus. To find the number of neutrons, you must first find the mass number. Here, Krypton’s mass number is 84 since its atomic weight, 83.80, rounds up to 84. The mass number is a count of the number of particles in an atom’s nucleus. What do we find in an atom’s nucleus??? PROTONS AND NEUTRONS!! So, • Mass Number= (#of Protons)+ (# of Neutrons) •84= 36+? The number of neutrons in an atom of krypton is 48 Summary… For any element, • Number of Protons= Atomic Number • Number of Electrons= Number of Protons=Atomic Number • Number of Neutrons= Mass Number-Atomic Number Electrons fill the energy levels outside the nucleus. Level Level Level Level Level Level 1 2 3 4 5 6 holds holds holds holds holds holds 2 electrons. 8 electrons. 18 electrons. 32 electrons. 50 electrons. 72 electrons. Energy levels do not have to be completely filled to start filling the next level. Let’s try some on our own… How many protons, neutrons, and electrons are found in each element? • Carbon (C) • Nitrogen (N) • Oxygen (O) • Phosphorus (P) • Calcium (Ca) • Hydrogen (H) Atomic Radius size of atom measured from center of nucleus to outside of electron cloud expressed in picometers (1012 pm = 1 m) usually 40-270 pm Example An atom has a radius of 140 pm. How large is that in meters? 1m 12 10 140 pm 12 140 10 1.4 10 m 10 pm Now, what is an element??? An element is a pure substance that cannot be broken down into other substances by physical or chemical means. Elements are made of only one type of atom. There are 118 known elements, 94 occur naturally. Isotopes Although atoms of the same element have the same number of protons and electrons, atoms of an element can have different numbers of neutrons. Atoms of the same element that have different numbers of neutrons are called isotopes. Compounds Elements can combine to form more complex substances. These substances are held together by chemical bonds (covalent and ionic.) A compound is a pure substance formed when two or more different elements combine. Compounds have several unique characteristics. Compounds are always formed from a specific combination of elements in a fixed ratio. Ex: H2O Compounds are chemically and physically different from the elements that form them. Compounds cannot be broken down into simpler compounds by physical means, such as crushing or tearing. Compounds can, however, be broken down by chemical means into simpler compounds or their original elements.