* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download 2/1: Atomic Structure
Survey
Document related concepts
Transcript
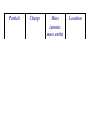
Atomic Structure- History Modern Day Scientists – Dalton • atoms could not be divided • all elements of a given element are the same • different atoms could join to form compounds – Thomson • the plum pudding model • negatively-charged "plums” surrounded by positivelycharged "pudding” – Rutherford http://www.mhhe.com/physsci/chemistry/essentialchemistry/flash/ruther14.swf • atom is made up of a central charge surrounded by a cloud of orbiting electrons – Bohr • electrons are in levels around the nucleus Next week Structure of the Atom Particle Charge Mass (atomic mass units) 1 Location Proton positive + Neutron neutral Ø 1 nucleus Electron negative - 0.0006 orbit, level, cloud nucleus • End here if did notes on same day of History of the Atom video. The Nucleus A 1946 test of an atomic bomb in the lagoon at Bikini atoll. The explosion has just started; surplus ships moored nearby can still be seen. The nucleus – the center of the atom composed of protons and neutrons – held together by four forces (electromagnetic, strong , weak, and gravity) – 99.9% of the atom’s mass is here – about 100,000 times smaller than the entire atom – the atomic number of an atom is the number of protons in the nucleus – the atomic mass or mass number of an atom is the sum of the protons and neutrons – Isotopes atoms with the same number of protons (and therefore the same element) but with a different number of neutrons.