* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Fire Up Your Atoms!!
Survey
Document related concepts
Transcript
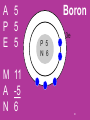
2-1 Review Atoms Warm - Up: ductility, malleability Today’s Agenda: 1. complete Atoms 1 (as group) 2. Review 3. Drawing Atomic Structure I - 3 -complete for HW Study for quiz on Friday--atoms September 29 45. malleability physical property of metals that lets them be flattened into thin sheets Draw it: Connect it: mallet jewelry blacksmith 2 46. ductility physical property of metals that lets them be twisted into wires Draw it: Connect it: jewelry electricity metals solids 3 Quick Review Atoms 4 Subatomic Particles PARTICLE CHARGE LOCATION PROTON MASS 1 0 NUCLEUS CLOUD 0 5 What particles are found in the nucleus of the atom? Protons and Neutrons they are help together by the STRONG FORCE 7 What particle is found surrounding the nucleus? What makes the atom neutral? Protons = Electrons + = - ( the charges, cancel each other out) 10 TO HELP ME FIND THE NUMBER OF SUBATOMIC PARTICLES IN ANY PARTICULAR ATOM, I have to remember the ACRONYM: 11 A P E M A N 12 A atomic # P protons (+) E elecrons (-) these 3 are the same # M mass number A atomic # N neutrons 13 Electrons are arranged in ____________________. 14 18e 8e 8e 2e P N Energy Levels 15 How many electrons are there? What is the atomic number? What is the number of protons? What is the atomic mass? Which element is this a model of? 21 A __ M __ P __ A -__ E __ N __ Don’t forget to round to a whole # 22 How many atoms are there? NaCl How many elements are there? NaCl How many atoms are there? CH4 How many elements are there? CH4 How many atoms are there? CaCO3 How many elements are there? CaCO3 Use the rest of the Class to Draw the Atoms of the elements on Atomic Structure I -III. These will be used to create your own Periodic Table this week. Do them NEATLY and remember to simplify. A 5 P 5 E 5 M 11 A -5 N 6 Boron 3e P 5 N 6 30