* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Trends on the Periodic Table
Survey
Document related concepts
Transcript
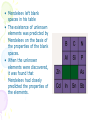
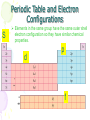
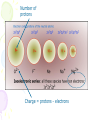
The History of the Modern Periodic Table During the nineteenth century, chemists began to categorize the elements according to similarities in their physical and chemical properties. The end result of these studies was our modern periodic table. Dmitri Mendeleev In 1869 he published a table of the elements organized by increasing atomic mass. 1834 1907 • Mendeleev left blank spaces in his table • The existence of unknown elements was predicted by Mendeleev on the basis of the properties of the blank spaces. • When the unknown elements were discovered, it was found that Mendeleev had closely predicted the properties of the elements. After the discovery of these unknown elements between 1874 and 1885, and the fact that Mendeleev’s predictions for Sc, Ga, and Ge were amazingly close to the actual values, his table was generally accepted. Periodic Law When elements are arranged in order of increasing atomic number, there is a periodic pattern in their physical and chemical properties. The periodic table is the most important tool in the chemist’s toolbox! Trends on the Periodic Table Brainiac: Alkali Metals Groups and Periods Groups or Families - a column of elements on the periodic table. There are 18 groups on the table Period - a row of elements on the periodic table. There are seven periods on the table Groups and Periods You need to know five family names and locations 18 1 2 3-12 17 Properties of Families Alkali metals (1)—The most reactive metal family, must be stored under oil because they react violently with water! They dissolve and create an alkaline, or basic, solution, hence their name. Alkaline earth metals (2)—These also are reactive metals, but they don’t explode in water; pastes of these are used in batteries. Halogens (17)—Known as the “salt formers,” they are used in modern lighting and always exist as diatomic molecules in their elemental form. Noble gases (18)—Known for their extremely low reactivity, these were once thought to never react; neon, one of the noble gases, is used to make bright signs. Periodic Table and Electron Configurations S Elements in the same group have the same outer shell electron configuration so they have similar chemical properties. d p f Metals, Nonmetals, Metalloids • There is a zig-zag or staircase line that divides the table. • Metals are on the left of the line, in blue. • Nonmetals are on the right of the line, in orange. Metals, Nonmetals, Metalloids • Elements that border the stair case, shown in purple are the metalloids or semimetals. • There is one important exception. • Aluminum is more metallic than not. Metals • Metals are lustrous (shiny), malleable, ductile, and are good conductors of heat and electricity. • They are mostly solids at room temp. • What is one exception? Nonmetals • Nonmetals are the opposite. • They are dull, brittle, nonconductors (insulators). • Some are solid, but many are gases, and Bromine is a liquid. Metalloids • Metalloids, aka semimetals are just that. • They have characteristics of both metals and nonmetals. • They are shiny but brittle. • And they are semiconductors. The Three Main Groups of Elements on the Periodic Table Metals • luster (shine) • good conductors of heat and electricity • solid at room temperature • most are malleable and ductile Non-metals • • • • Dull, not shiny poor conductors of heat and electricity neither malleable or ductile many are gasses at room temperature Semimetals or metalloids • have some properties of metals and some of non-metals Trends on the Periodic Table Atomic Radius – the size (diameter) of the atom Decreases as you go across a period, but increases as you go down a group. Ionization energy – the energy required to remove a valence electron. (outer most electron) Increases as you go across a period, but decreases as you go down a group. Trends Decreasing Ionization Energy Increasing ionization energy Why the Trend in Atomic Radius? Across a period the radius decreases• As you go from left to right the protons in the nucleus increase and the electrons also increase which causes the atom to be 'sucked' together a little tighter. Down a group radius increases • A new energy level of electrons is added to the atom as you go down each row, making each atom significantly larger. Why the Trend in Ionization Energy? Increases as you go across a group because the elements are getting smaller and the electrons are closer to the nucleus making it harder to remove an electron. Decreases as you go down a column because the electrons are further from the nucleus and easier to remove. (lower ionization energy) Ionic size • Metallic elements easily lose electrons. • Non-metals more readily gain electrons. How does losing or gaining an electron effect the size of the atom (ion) ? Positive ions • Positive ions are always smaller that the neutral atom. Loss of outer shell electrons. Negative Ions • Negative ions are always larger than the neutral atom. Gaining electrons. Number of protons Charge = protons - electrons Ion size trends in columns. • Ion size increases as you move down a column for both positive and negative ions