* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download File
Saturated fat and cardiovascular disease wikipedia , lookup
Cardiovascular disease wikipedia , lookup
Cardiac contractility modulation wikipedia , lookup
History of invasive and interventional cardiology wikipedia , lookup
Lutembacher's syndrome wikipedia , lookup
Remote ischemic conditioning wikipedia , lookup
Electrocardiography wikipedia , lookup
Mitral insufficiency wikipedia , lookup
Heart failure wikipedia , lookup
Hypertrophic cardiomyopathy wikipedia , lookup
Antihypertensive drug wikipedia , lookup
Quantium Medical Cardiac Output wikipedia , lookup
Cardiac surgery wikipedia , lookup
Jatene procedure wikipedia , lookup
Dextro-Transposition of the great arteries wikipedia , lookup
Arrhythmogenic right ventricular dysplasia wikipedia , lookup
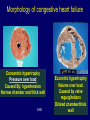
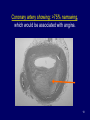
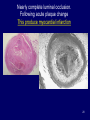
The Heart -1 Updated for Spring 2008 Dr. Amitabha Basu MD 1 Topic • Heart failure • Ischemic heart disease 2 Congestive heart failure • Def: reduced cardiac output to meet the demand. • In many pathologic states, the onset of heart failure is preceded by cardiac hypertrophy. • Pathogenesis: flow chart next slide. 3 Flow chat Increased work load, MI, pressure/ volume overload ↓ re-expression of embryonic/fetal type of protein (β-myosin heavy chain) + Reduced capillary density → reduced oxygen supply ↓ Heart failure These re-expression of embryonic/fetal type are associated with myocardial hypertrophy. 4 Congestive heart failure • So, it is long term process. • Clinical: similar to RHF • Often progress from the underling diseases like – Hypertension – Cor-pulmonale – Valvular disease – Multiple MI 5 Morphology of congestive heart failure Concentric hypertrophy Pressure over load Caused By: hypertension Narrow chamber and thick wall END Eccentric hypertrophy Volume over load. Caused by valve regurgitations Dilated chamber/thick wall 6 Left Heart failure • Left heart failure: etiology – Ischemic heart disease – Hypertension – Aortic / Mitral valve disease • Presentation: acute onset of dyspnea, pulmonary edema, rales, S3 gallop. • Complication: Cardiogenic shock 7 Pulmonary congestion and edema. Intraalveolar pale pink, low protein, few lymphocytes fluid: Transudate) 8 Right heart failure • Etiology: – Left heart failure and Cor-pulmonale • Clinical: – jugular venous distension, hepatoslemegaly, dependent edema, ascites, pleural effusion. • Complication: – Chronic passive congestion of liver (nutmeg liver) – Cardiac cirrhosis and centrilobular necrosis. 9 A classic case of progression of heart failure • Next slide 10 LVF ↓ Dyspnea ← Pul. Edema (T) + heart failure cells ← ↑Hydrostatic pr. ←PHT ↓ reduced lung edema ← ↓ Flow of blood in the lung ←RHF ↓ Increased (central) venous pressure ↓ ↑ Hydrostatic pressure in peripheral blood vessels in the soft tissue = Pitting (dependent edema) + Ascitis + Develop Passive venous congestion of various organs Liver: hepatomegaly (Nutmeg liver)/cardiac cirrhosis Spleen: congestive splenomegaly Kidney : Hypoxia (Sec. hypertension) PHT- pulmonary hypertension 11 Ischemic Heart Disease (IHD) Dr. Basu MD 12 Do you know • Propels over 6000 liters of blood through the body daily. • Beats more than 40 million times a year. • Yearly economic burden of ischemic heart disease is estimated to be in excess of $100 billion. 13 Topic • • • • Definition Types of IHD Pathogenesis of Ischemic Heart Disease Myocardial infarction 14 Ischemic Heart Disease • There is an imbalance between the myocardial demand and the blood supply. • Age: Male: middle age – Female : post menopausal • Epidemiology: leading cause of death for both males and females in the United States. 15 Types of ischemic heart disease 1. Angina ( with > 75% narrowing) 1. Angina pectoris (classical, stable angina) 2. Prinzmetal variant 3. Unstable angina Acute Coronary Syndrome 2. Myocardial infarction (100% occlusion) 3. Sudden cardiac death 4. Chronic ischemic heart disease 16 Pathogenesis of IHD 1. Narrowing (stenosis) of coronary artery: • Mostly fixed arthrosclerosis. 2. Complete obstruction (occlusion) of the lumen of coronary artery: 1. Thrombus developed on an atheroma / embolism**. 1. 75% or more narrowing: Ischemic symptoms (angina). 2. Complete occlusion (100% ): Infarction 17 Morphology- Angina • Fixed Atherosclerotic narrowing of the coronary artery. • This partial occlusion may produce angina. 18 Coronary artery showing: >75% narrowing, which would be associated with angina. 19 Nearly complete luminal occlusion. Following acute plaque change This produce myocardial infarction 20 Angina Pectoris • Angina pectoris is characterized by: – • Intermittent chest pain caused by transient, reversible myocardial ischemia. Type: 1. Typical or stable angina pectoris. • Fixed atherosclerotic narrowing (75% or greater). 2. Prinzmetal, or variant , angina. 3. Unstable angina pectoris (crescendo angina). • Preinfarction angina. 21 Typical or stable angina pectoris • Episodic chest pain associated with exertion or stress. • Pathogenesis: Fixed coronary atherosclerotic narrowing without plaque change ( > 75% but not full) • Pain is relived by rest or vasodilators (nitroglycerine) that reduce the venous return. 22 Prinzmetal Angina • Is a form of angina pectoris which – occurs at rest and – presumably stems from a coronary artery spasm with or without an obstructive lesion in the artery. 23 Unstable Angina (crescendo angina) • Pathogenesis: – acute plaques change but without 100% occlusion. • Clinical: – Increased frequency of anginal pain. – Pain precipitated by less exertion. – Pain is more intense and last longer. • High risk for Myocardial infarction. 24 Biochemical of angina • C-reactive protein (CRP) may serve as a marker to predict the risk of MI in patients with angina. • Also serve as a marker to predict the risk of new infarcts in patients who recover from infarcts. 25 Myocardial Infarction 26 Myocardial infarction Sudden (> 30 min.) pain May be in shock due acute LVF : Pulmonary edema. Statistics: In U.S. 1.5 million/yr. 25% die in acute phase within 1 hr. At age 45 –55 : M:F::4:1 At age 80 equal incidence among sexes 27 Pathogenesis of Myocardial Infarction – 100% Occlusive intracoronary thrombus By:1. Provoked by complications of Atheroma: E.g. : Rupture of plaque. 2. Coronary artery spasm: ? Smoking 3. Emboli (Source is the proximal part of same blood vessels). 28 Types of Myocardial Infarction • Subendocardial MI: – < 50% of the wall thickness – Any MI typically begins in this area (most poorly perfused area of the myocardium). • EKG: ST segment depression. • Transmural MI: – Necrosis extend externally and involve entire myocardium. – Most common type, take about 24 hrs to develop. 29 M.I: Sites of occlusion:infarction • LAD = Lt. Ant. Desc. A (40 –50%):– Lt.Ventricle : anterior and apical – Ant.2/3 of Inter Ventricular Septum (IVS) • RCA= Rr. Coronary A (30 40%) – Lt.Ventricle : post. wall – I.V.S.post.1/3 • LCX= Lt. Circumflex A(15-20%):– Lt.Ventricle -- lateral wall 30 LAD occlusion Right Coronary occlusion Left Circumflex occlusion 31 Reversible Injury Time Gross Features 0-½ hr None Morphology Light Electron Microscope Microsco pe None Relaxation of myofibrils; glycogen loss; mitochondrial swelling. 32 Morphology of Myocardial Infarction Time from Onset Gross Morphologic Finding 18 - 24 Hours Pallor of myocardium 3 - 7 Days Hyperemic border with central yellowing Maximally yellow and soft with vascular margins White fibrosis 10 - 21 Days 7 weeks 33 Acute myocardial infarct, predominantly of the posterolateral left ventricle SCAR 34 Time from Onset 0-30 min. Microscopic Finding 1-3 hours No change (E.M- Mitochondrial swelling) Irreversible cell death ↓ Few wavy fibers at the margin of MI. 4-12 hours Loss of cross striations and edema. 12-24 hours Coagulative necrosis, Marginal contraction bands necrosis, ( and PMNs infiltrate). 35 Time from Onset Microscopic Finding 24 - 72 Hours 10-21 Days Plenty of PMNs and coagulation necrosis Macrophage & mononuclear infiltration prominent granulation tissue 7-8 Wk Fibrosis: Healing 4-7 days 36 1 to 3 hours wavy fibers (elongated and narrow), compared with adjacent normal fibers (at right). 37 12 -24 hrs. Contraction band necrosis : Note the many irregular darker pink wavy contraction bands extending across the fibers. CONTRACTION BANDS 38 Remote infarction ( left Trichrome stain: showing blue collagen). 39 MECHANISM of reperfusion injury • Generation of oxygen free radicals from infiltrating leukocytes • And apoptosis. 40 Effect of reperfusion injury 41 Biochemistry of MI Elevated by Peak MYOGLOBIN may rise immediately CK- MB 4-8 hr 18-20hr Return to normal 2-3 days CK-MB Isoform 1 & 2 Normal ratio of 1 : 2 = 1.2 In MI the ratio of 1: 2 is >1.5 42 Biochemistry of MI Elevated Peak by 3-6 hr 16-20hr Cardiac specific Troponin I and T LDH 24hr ( level depends on the amount of cell death) 3-6 days Return to normal 7-10 days 8-14 days In MI, LDH “Flip” occur. Normally LDH2 > LDH1, After MI : LDH1 > LDH2 43 Enzymes 44 Complications of MI Arrhythmias with possible "sudden death” (ventricular fibrillation) Acute fibrinous pericarditis Rupture of the wall and tamponade or VSD Papillary muscle rupture and mitral incompetence Occur immediately [ MOST COMMON cause of sudden death in MI] Typical onset: Second or third day Late: Dressler syndrome 3-7 days after the MI 3-7 days after the MI 45 Cardiac tamponade Occur due to hemopericardium. ↑ pericardial pressure = ↓ diastolic filling of the ventricles, and hence in stroke volume Signs: Sudden drop in systolic and well as diastolic blood pressure. Distended jugular vein. Right ventricular and right atrial collapse. 46 OTHERS complications Mural thrombi and thrombo-embolism: Infarct of Brain, kidney, Intestine 1wk or > Carcinogenic shock (10% cases) Multi-organ failure MI is the most common cause of Cardiogenic shock. Extension of infarct or repeat 2nd Any time and the most infarction- ? diagnosis common cause of sudden death weeks after an MI Ventricular aneurysms Late complication 47 Myocardial rupture in an acute infarct in the wall : ? consequence Hemopericardium Cardiac tamponade:Acute chest pain/ sudden drop of BP 48 Rupture of the ventricular septum (L) and papillary muscle (R) Left-to-right shunt and right heart failure. Produce sudden mitral insufficiency. Pan systolic murmur Holosystolic murmur (laterally at the apex of the heart with the patient in the left lateral decubitus position) radiated to axilla . 49 left ventricular aneurysm: does not contract , so the ejection fraction and stroke volume of the heart are reduced- patient feels week!. 50 Clinical Features of Myocardial Infarction • Severe, crushing substernal chest pain. • Pain radiate to either: – Neck ,Epigastrium,Shoulder. – Or left arm. • In up to 50% of cases, pain is preceded by episodes of angina pectoris. 51 Diagnosis of Myocardial Infarction – EKG – ST elevation changes, T wave inversion and Q wave- transmural infarct. – Echo cardiogram – Myocardial enzyme markers • Creatinine Kinase • Troponins • Lactate dehydrogenase, Myoglobin 52 Sudden Cardiac Death (SCD) Mechanism of SCD is most often a lethal arrhythmia . Commonest cause = I.H.D Other causes:2. Aortic stenosis 3. 2nd Myocardial infarct 4. M.V. prolapse 5. Cardiomyopathy 6. Myocarditis 53 Thank you 54