* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download CorePace #5 - Pacemaker Basics
Remote ischemic conditioning wikipedia , lookup
Heart failure wikipedia , lookup
Jatene procedure wikipedia , lookup
Cardiac surgery wikipedia , lookup
Myocardial infarction wikipedia , lookup
Hypertrophic cardiomyopathy wikipedia , lookup
Management of acute coronary syndrome wikipedia , lookup
Cardiac contractility modulation wikipedia , lookup
Quantium Medical Cardiac Output wikipedia , lookup
Ventricular fibrillation wikipedia , lookup
Arrhythmogenic right ventricular dysplasia wikipedia , lookup
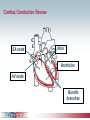
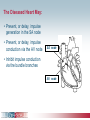
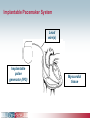
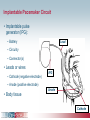
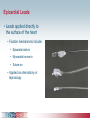
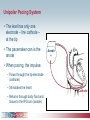
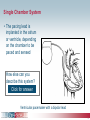
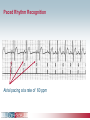
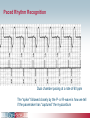
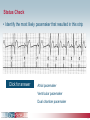
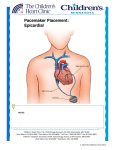
Pacemaker Basics Module 5 1 Objectives • Identify the components of a pacemaker circuit • Identify how pacemaker leads and systems are characterized • Perform basic pacemaker rhythm recognition 2 Intrinsic Pacemaker • The heart generates electrical impulses that travel along a specialized conduction pathway – Typically, the sinus node (SA node) generates the impulses – Conduction paths include: • Bachman’s Bundle • AV node • Bundle of HIS • Bundle Branches • Purkinje Network • This pattern of conduction makes it possible for the heart to pump blood efficiently Cardiac Conduction Review SA node Atria Ventricles AV node Bundle branches The Diseased Heart May: • Prevent, or delay, impulse generation in the SA node • Prevent, or delay, impulse conduction via the AV node SA node • Inhibit impulse conduction via the bundle branches AV node Implantable Pacemaker System Lead wire(s) Implantable pulse generator (IPG) Myocardial tissue Implantable Pacemaker Circuit • Implantable pulse generator (IPG): – Battery Lead – Circuitry – Connector(s) • Leads or wires – Cathode (negative electrode) IPG – Anode (positive electrode) • Body tissue Anode Cathode The Pulse Generator • Contains a battery to provide energy for sending electrical impulses to the heart • Houses the circuitry that controls pacemaker operations • Includes a connector to join the pulse generator to the lead(s) Connector Block Circuitry Battery Leads are Insulated Wires • Deliver electrical impulses from the pulse generator to the heart • Sense cardiac depolarization Lead Lead Characterization • Position within the heart • Polarity – Endocardial or transvenous leads – Unipolar – Epicardial leads – Bipolar • Fixation mechanism • Insulator – Active/Screw-in – Silicone – Passive/Tined – Polyurethane • Shape – Straight – J-shaped used in the atrium Endocardial Passive Fixation Leads • The tines become lodged in the trabeculae, a fibrous meshwork, of the heart • Inserted via a cut-down or transvenous sheath Tines Transvenous Active Fixation Leads • The helix, or screw, extends into the endocardial tissue – Allows for lead positioning anywhere in the heart’s chamber – The helix is extended using an included tool – Inserted via a cut-down or transvenous sheath Epicardial Leads • Leads applied directly to the surface of the heart – Fixation mechanisms include: • Epicardial stab-in • Myocardial screw-in • Suture-on – Applied via sternotomy or laproscopy Lead Polarity • Unipolar leads – May have a smaller diameter lead body than bipolar leads Unipolar lead – Usually exhibit larger pacing artifacts on the surface ECG To tip (cathode) • Bipolar leads – Usually less susceptible to oversensing of non-cardiac signals (i.e., myopotentials, EMI, etc.) Bipolar coaxial lead Unipolar Pacing System • The lead has only one electrode – the cathode – at the tip • The pacemaker can is the anode Anode + • When pacing, the impulse: – Flows through the tip electrode (cathode) – Stimulates the heart – Returns through body fluid and tissue to the IPG can (anode) Cathode - 15 Bipolar Pacing System • The lead has both an anode and cathode • The pacing impulse: – Flows through the tip electrode located at the end of the lead wire – Stimulates the heart Anode + – Returns to the ring electrode, the anode, above the lead tip Anode Cathode - Cathode Lead Insulators Silicone insulated leads • Inert • Biocompatible • Biostable • Repairable with medical adhesive Polyurethane Silicone • Historically very reliable Polyurethane insulated leads • Biocompatible • High tear strength • Low friction coefficient • Smaller lead diameter Newer bipolar lead insulation Single Chamber and Dual Chamber Pacing Systems Single Chamber System • The pacing lead is implanted in the atrium or ventricle, depending on the chamber to be paced and sensed How else can you describe this system? Click for answer Ventricular pacemaker with a bipolar lead 19 Paced Rhythm Recognition Atrial pacing at a rate of 60 ppm Paced Rhythm Recognition Ventricular pacing at a rate of 60 ppm The “spike” is an artifact created by the pacemaker’s output Paced Rhythm Recognition On the ECG, note the difference between the paced and intrinsic beats. Why do you think this is? Click for answer Because the paced beat originates and is conducted differently than the intrinsic beat. 22 Advantages/Disadvantages of Single Chamber Pacing Systems • Advantages – Implant only one lead – Pacemaker itself usually smaller • Disadvantages – Single ventricular lead does not provide AV synchrony • Ventricular based pacing linked to AF and HF hospitalizations – Single atrial lead does not provide ventricular backup if A-V conduction is lost Dual Chamber System • Two leads – One lead implanted in the atrium – One lead implanted in the ventricle • These systems can be unipolar or bipolar Are these leads in the picture active or passive fixation? Click for answer Passive fixation. Note, the tines look like small grappling hooks, but are actually soft silicone. Paced Rhythm Recognition Dual chamber pacing at a rate of 60 ppm The “spike” followed closely by the P- or R-wave is how we tell if the pacemaker has “captured” the myocardium Why a Pacemaker is Implanted • A pacemaker is implanted to: – Provide a heart rate to meet metabolic needs • In order to pace the heart, it must capture the myocardium • In order to pace the heart, it must know when to pace, i.e., it must be able to sense – Today’s pacemakers also: • Provide diagnostic information – About the pacing system – About the patient We’ll discuss how a pacemaker performs these functions in upcoming CorePace modules. 26 Status Check • Which beats are paced? 1 2 Click for answer 3 4 5 6 7 Beats 3 and 6 are intrinsic 27 Status Check • Is this a unipolar or bipolar lead? Click for answer Bipolar. The tip (screw) is the cathode, the ring is the anode. For simplicity’s sake we often refer to these as the “tip” and “ring.” 28 Status Check In this unipolar system, the lead tip is negatively charged (cathode). What acts as the positive electrode (anode)? Anode + Click for answer Cathode - The pacemaker case. 29 Status Check Provide an explanation for why the pacemaker did not “capture” (think of the electrical concepts) ? Click for answer Lead fracture, failing battery, improper programming, insulation failure any or all might explain this. We’ll discuss how to determine the cause in “Evaluation and Troubleshooting.” 30 Status Check • Identify the most likely pacemaker that resulted in this strip Click for answer Atrial pacemaker Ventricular pacemaker Dual chamber pacemaker 31 Brief Statements Indications • Implantable Pulse Generators (IPGs) are indicated for rate adaptive pacing in patients who ay benefit from increased pacing rates concurrent with increases in activity and increases in activity and/or minute ventilation. Pacemakers are also indicated for dual chamber and atrial tracking modes in patients who may benefit from maintenance of AV synchrony. Dual chamber modes are specifically indicated for treatment of conduction disorders that require restoration of both rate and AV synchrony, which include various degrees of AV block to maintain the atrial contribution to cardiac output and VVI intolerance (e.g. pacemaker syndrome) in the presence of persistent sinus rhythm. • Implantable cardioverter defibrillators (ICDs) are indicated for ventricular antitachycardia pacing and ventricular defibrillation for automated treatment of life-threatening ventricular arrhythmias. • Cardiac Resynchronization Therapy (CRT) ICDs are indicated for ventricular antitachycardia pacing and ventricular defibrillation for automated treatment of life-threatening ventricular arrhythmias and for the reduction of the symptoms of moderate to severe heart failure (NYHA Functional Class III or IV) in those patients who remain symptomatic despite stable, optimal medical therapy and have a left ventricular ejection fraction less than or equal to 35% and a QRS duration of ≥130 ms. • CRT IPGs are indicated for the reduction of the symptoms of moderate to severe heart failure (NYHA Functional Class III or IV) in those patients who remain symptomatic despite stable, optimal medical therapy, and have a left ventricular ejection fraction less than or equal to 35% and a QRS duration of ≥130 ms. Contraindications • IPGs and CRT IPGs are contraindicated for dual chamber atrial pacing in patients with chronic refractory atrial tachyarrhythmias; asynchronous pacing in the presence (or likelihood) of competitive paced and intrinsic rhythms; unipolar pacing for patients with an implanted cardioverter defibrillator because it may cause unwanted delivery or inhibition of ICD therapy; and certain IPGs are contraindicated for use with epicardial leads and with abdominal implantation. • ICDs and CRT ICDs are contraindicated in patients whose ventricular tachyarrhythmias may have transient or reversible causes, patients with incessant VT or VF, and for patients who have a unipolar pacemaker. ICDs are also contraindicated for patients whose primary disorder is bradyarrhythmia. 32 Brief Statements (continued) Warnings/Precautions • Changes in a patient’s disease and/or medications may alter the efficacy of the device’s programmed parameters. Patients should avoid sources of magnetic and electromagnetic radiation to avoid possible underdetection, inappropriate sensing and/or therapy delivery, tissue damage, induction of an arrhythmia, device electrical reset or device damage. Do not place transthoracic defibrillation paddles directly over the device. Additionally, for CRT ICDs and CRT IPGs, certain programming and device operations may not provide cardiac resynchronization. Also for CRT IPGs, Elective Replacement Indicator (ERI) results in the device switching to VVI pacing at 65 ppm. In this mode, patients may experience loss of cardiac resynchronization therapy and / or loss of AV synchrony. For this reason, the device should be replaced prior to ERI being set. Potential complications • Potential complications include, but are not limited to, rejection phenomena, erosion through the skin, muscle or nerve stimulation, oversensing, failure to detect and/or terminate arrhythmia episodes, and surgical complications such as hematoma, infection, inflammation, and thrombosis. An additional complication for ICDs and CRT ICDs is the acceleration of ventricular tachycardia. • See the device manual for detailed information regarding the implant procedure, indications, contraindications, warnings, precautions, and potential complications/adverse events. For further information, please call Medtronic at 1-800-328-2518 and/or consult Medtronic’s website at www.medtronic.com. Caution: Federal law (USA) restricts these devices to sale by or on the order of a physician. 33 Brief Statement: Medtronic Leads Indications • Medtronic leads are used as part of a cardiac rhythm disease management system. Leads are intended for pacing and sensing and/or defibrillation. Defibrillation leads have application for patients for whom implantable cardioverter defibrillation is indicated Contraindications • Medtronic leads are contraindicated for the following: • ventricular use in patients with tricuspid valvular disease or a tricuspid mechanical heart valve. • patients for whom a single dose of 1.0 mg of dexamethasone sodium phosphate or dexamethasone acetate may be contraindicated. (includes all leads which contain these steroids) • Epicardial leads should not be used on patients with a heavily infracted or fibrotic myocardium. • The SelectSecure Model 3830 Lead is also contraindicated for the following: • patients for whom a single dose of 40.µg of beclomethasone dipropionate may be contraindicated. • patients with obstructed or inadequate vasculature for intravenous catheterization. 34 Brief Statement: Medtronic Leads (continued) Warnings/Precautions • People with metal implants such as pacemakers, implantable cardioverter defibrillators (ICDs), and accompanying leads should not receive diathermy treatment. The interaction between the implant and diathermy can cause tissue damage, fibrillation, or damage to the device components, which could result in serious injury, loss of therapy, or the need to reprogram or replace the device. • For the SelectSecure Model 3830 lead, total patient exposure to beclomethasone 17,21-dipropionate should be considered when implanting multiple leads. No drug interactions with inhaled beclomethasone 17,21-dipropionate have been described. Drug interactions of beclomethasone 17,21-dipropionate with the Model 3830 lead have not been studied. Potential Complications • Potential complications include, but are not limited to, valve damage, fibrillation and other arrhythmias, thrombosis, thrombotic and air embolism, cardiac perforation, heart wall rupture, cardiac tamponade, muscle or nerve stimulation, pericardial rub, infection, myocardial irritability, and pneumothorax. Other potential complications related to the lead may include lead dislodgement, lead conductor fracture, insulation failure, threshold elevation or exit block. • See specific device manual for detailed information regarding the implant procedure, indications, contraindications, warnings, precautions, and potential complications/adverse events. For further information, please call Medtronic at 1-800-328-2518 and/or consult Medtronic’s website at www.medtronic.com. Caution: Federal law (USA) restricts this device to sale by or on the order of a physician. 35 Disclosure NOTE: This presentation is provided for general educational purposes only and should not be considered the exclusive source for this type of information. At all times, it is the professional responsibility of the practitioner to exercise independent clinical judgment in a particular situation. 36