* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Rhythm management devices
Heart failure wikipedia , lookup
Management of acute coronary syndrome wikipedia , lookup
Myocardial infarction wikipedia , lookup
Lutembacher's syndrome wikipedia , lookup
Cardiac surgery wikipedia , lookup
Hypertrophic cardiomyopathy wikipedia , lookup
Cardiac contractility modulation wikipedia , lookup
Ventricular fibrillation wikipedia , lookup
Quantium Medical Cardiac Output wikipedia , lookup
Atrial fibrillation wikipedia , lookup
Electrocardiography wikipedia , lookup
Arrhythmogenic right ventricular dysplasia wikipedia , lookup
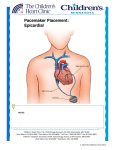
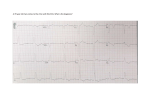
Rhythm Management Devices Tatyana Travkina, MD and Rosario Garcia, MD Overview • Increased number of patients with implanted cardiac devices • Uncomfortable / lack of knowledge • The always seems to be... …To Magnet or Not to Magnet? History: 1700’s De facto cardiac electrostimulation began in the mideighteenth century with the use of currents from the Leyden jar or Voltaic Pile (Allessandra Volta, 1799) to stimulate cardiac nerves and muscles in animals and to attempt resuscitation of intact dead animals. Dr. William Hawes in London established The Humane Society of London in 1774 AD - A fraternity devoted to salvaging persons seemingly dead - motivated by similar such society in Paris. Later on it became The Royal Humane Society of London. Squires, Henley and Fothergill suggested Electrostimulation for resuscitation in a number of communications to the Society between 1774 and 1748. Such an incidence is described by Charles Kite in his “An Essay upon the Recovery of the Apparently Dead.” (London, 1788) The 3year old child was taken up for dead after falling out of a window. An “apothecary” was sent for, who could nothing; then electrical resuscitation by an electrostatic generator with a Leyden jar capacitor was used. (Fig.1) History: 1800’s • In 1802, Nysten used a human cadaver shortly following death by execution to demonstrate that the ability to reactivate the heart electrically was lost earlier for the left ventricle, later for the right ventricle, still later for the left atrium and last for right atrium. • Later in the nineteenth century Althaus (1864) reported successful resuscitation of cardiac arrest victims by electrical currents applied through transthoracic needle.[ • Dr.DeSanctis used “Re-animation Chair.”(Fig.2) as described by Richmond Reece in his The Medical Guide (1820). It had 3 pertinent features: a bellows to give forced ventilation, a metallic tube to be inserted into the esophagus and a voltaic pile attached at one pole to the esophageal tube and at the other to an electrode. The electrode was to be successively touched to “the regions of the heart, the diaphragm and the stomach…” These reports may have lead John Hunter to recommend in 1776 that electrostimulation be tried as the as the resort in the resuscitation of drawing victims. History: 1900’s • In 1899, Prevost and Battelli demonstrated that electrical currents could cause ventricular fibrillation that often could be reversed by another powerful stimulus of either alternating or direct current. • Robinvitch in a series of reports from 1907 through 1909 confirmed this work and designed the first portable electrical resuscitative apparatus for ambulances. • MacWilliam, in many publications beginning in 1899 and extending to World War I, further elucidated the pathophysiology of ventricular fibrillation and described deterioration of cardiac pump function by tachyarrhythmias as well as bradarrythmias. History: 1930’s • In 1932, Albert Hyman developed a machine for controlled repetitive electrostimulation of heart and named his device the “artificial cardiac pacemaker.” [26] (Fig.3) In Hyman’s Words: • “Finally on April 6,1930, I received Grant No. 30-2 from the Witkin Foundation to explore the possibility of developing a practical machine, to be used as an artificial pacemaker in experimental animals.” History: 1950’s • In 1958, engineer Earl Bakken of Minneapolis, Minnesota, produced the first wearable external pacemaker for a patient of Dr. C. Walton Lillehei. This transistorised pacemaker, housed in a small plastic box, had controls to permit adjustment of pacing heart rate and output voltage and was connected to electrode leads which passed through the skin of the patient to terminate in electrodes attached to the surface of the myocardium of the heart. History: 1950’s • The first clinical implantation into a human of a fully implantable pacemaker was in 1958 at the Karolinska Institute in Solna, Sweden, using a pacemaker designed by Rune Elmqvist and surgeon Åke Senning. • It connected to electrodes attached to the myocardium of the heart by thoracotomy. The device failed after three hours. A second device was then implanted which lasted for two days. • The world's first implantable pacemaker patient, Arne Larsson, went on to receive 26 different pacemakers during his lifetime. He died in 2001, at the age of 86, outliving the inventor as well as the surgeon. Physiology • Delivery of electrical current (< 1.0 ms, <3.0 V) via an insulated pacing lead to the heart muscle at a preprogrammed rate. • The wires in a single-chamber pacemaker usually carry pulses from the generator to the right ventricle • The wires in a dual-chamber pacemaker carry pulses from the generator to the right atrium and the right ventricle. The pulses help coordinate the timing of these two chambers' contractions. • The wires in a biventricular pacemaker carry pulses from the generator to the right atrium and both ventricles. The pulses help coordinate electrical signaling between the two ventricles. This type of pacemaker also is called a cardiac resynchronization therapy. Indications for Permanent Pacemaker Insertion- Bradycardia Class I - There is evidence and/or general agreement that permanent pacing is indicated in patients with sinus node dysfunction in the following settings: • Documented symptomatic bradycardia, including frequent sinus pauses that produce symptoms. (Level of Evidence: C) • Symptomatic chronotropic incompetence. (Level of Evidence: C) • Symptomatic sinus bradycardia that results from required drug therapy for medical conditions. (Level of Evidence: C) Class IIa - The weight of evidence or opinion is in favor of the usefulness of permanent pacing in patients with sinus node dysfunction in the following settings: • Heart rate <40 beats per minute when a clear association between significant symptoms consistent with bradycardia and the actual presence of bradycardia has not been documented. (Level of Evidence: C) • Syncope of unexplained origin when clinically significant abnormalities of sinus node function are discovered or provoked in electrophysiological studies. (Level of Evidence: C) Class IIb - The evidence or opinion is less well established that permanent pacing in sinus node dysfunction is beneficial in the following setting: • Minimally symptomatic patients with chronic heart rate <40 beats per minute while awake. (Level of Evidence: C) Class III - There is evidence that permanent pacing in sinus node dysfunction is not useful and may be harmful in the following settings: • Asymptomatic patients. (Level of Evidence: C) • Patients for whom the symptoms suggestive of bradycardia have been clearly documented to occur in the absence of bradycardia. (Level of Evidence: C) • Symptomatic bradycardia due to nonessential drug therapy. (Level of Evidence: C) Indications for Permanent Pacemaker Insertion- AV block Indications for Permanent Pacemaker Insertion- post-MI Pacemaker mode codes Table 1 The North American Pacing and Electrophysiology/British Pacing and Electrophysiology Group (NASPE/BPEG) pacemaker codes: Position I Position II Position III Position IV Position V Pacing chamber Sensing chamber Response to sensing Programmabilit Antiy tachycardia functions 0=none 0=none 0=none 0=none 0=none A=atrium A=atrium I=inhibited R=rate modulation P=pace V=ventricle V=ventricle T=triggered S=shock D=dual (atrium and ventricle) D=dual (atrium and ventricle) D=dual (inhibited and triggered) ) D=dual (pace and shock) Ventricular Pacing: Fixed-Rate and Demand Pacemakers I. The two modes of pacemaker function are fixed rate and demand. I. A fixed-rate pacemaker is one that fires at a specific preset rate, regardless of the patient's own heart rate. II. A demand pacemaker literally functions "on demand" (i.e., only when the patient's heart rate falls below a preset value). A demand pacemaker has two distinct aspects: I. a sensing mechanism designed so that the pacemaker is inhibited when the patient's own heart rate is adequate II. a pacing mechanism designed to trigger the pacemaker when no intrinsic QRS complexes occur within a predetermined period. III. Demand units can be temporarily converted to fixed-rate mode by placing a special magnet on the chest wall over the battery. The magnet test is routinely performed when the pacemaker rate is being checked. IV. Present-day demand-type pacemakers are also "programmable." This means that the pacing rate can be adjusted once a pacemaker is implanted. Adjustment of rate is accomplished by placing a special telemetry device on the chest wall to allow communication with the pacemaker. V. Demand ventricular pacemakers usually are QRS (R wave) inhibited. They emit pulses only when the spontaneous heart rate falls below the escape rate of the pacemaker (e.g., 70 beats/min) They do not emit pulses when the patient's spontaneous heart rate is faster than the escape rate of the pacemaker. Each time a QRS inhibited pacemaker senses a spontaneous QRS complex, formation of a pacemaker pulse is inhibited. Ventricular Pacing: Fixed-Rate and Demand Pacemakers I. The QRS-inhibited pacemaker also has a refractory period (e.g., 0.4 second) that begins whenever the pacemaker senses a QRS complex or emits a pulse. I. During the refractory period the pacemaker cannot sense another R wave. However, if spontaneous ventricular beats or ventricular premature complexes occur during the refractory period, they are not inhibited but appear on the ECG. II. The refractory period is followed by an alert period, during which the pacemaker can sense an R wave. If no QRS complex is sensed by the end of the alert period, the pacemaker emits another pulse. I. However, if a spontaneous QRS complex occurs, the pacemaker is recycled into another refractory period and then into another alert period. Dual-Chamber Pacing I. In dual-chamber pacemakers, electrodes are inserted into both the right atrium and right ventricle. II. The atrial lead is able to sense the patient's intrinsic P waves and stimulates the atrium when the atrial rate becomes too slow. I. The circuitry is designed to allow for a physiologic delay between atrial and ventricular stimulation. This atrioventricular (AV) delay is analogous to the PR interval seen in normal conduction. III. Dual-chamber pacing is helpful in maintaining physiologic timing between atrial and ventricular systole. I. When ventricular pacing alone is used, this physiologic timing is lost. In some patients the loss of timed atrial contractions causes a marked reduction in cardiac output. II. Dual-chamber pacing produces a significant improvement in the cardiac performance of these individuals. In selected patients, dual-chamber pacing may allow a physiologic increase in ventricular rate during exercise. Pacemaker mode codes-Example • • • • • You have a patient in 3rd degree heart block with a pacemaker in DDD mode. What does that mean? The first D in DDD is the area PACED. The D stands for DUAL, so this pacemaker, paces both the atrium and the ventricle. The second letter stands for Area SENSED, so this pacemaker senses both the atrium and the ventricle. The third letter stands for what it does, and the D stands for Dual inhibited. This pacemaker can be INHIBITED by: • Electrical Noise Caused by Electro cautery • Refractory Period electrical activity from an atrial contraction and it will not pace the atrium. • electrical activity of a ventricular contraction, and not pace the ventricle, or both. Rate-adaptive/rate-modulated pacing I. normal heart rate response to increased physiological demand is linearly related to oxygen demand/consumption II. greater proportion of rate-adaptive sensors belong to a secondary class of sensors that detect physiological changes as a consequence of exercise such as: I. such as QT interval shortening II. increase in respiratory or minute-ventilation rate III. increased mean atrial rate IV. rise in central venous temperature V. decrease in venous blood pH VI. increase in right ventricular stroke volume VII. increase in ventricular inotropy (e.g. peak endocardial acceleration and ventricular impedence variation). Questions 1.Which of the following is the major indication for a permanent pacemaker? a.History of multiple prior myocardial infarctions b.Symptomatic bradyarrhythmia c.Digitalis toxicity d.Ventricular bigeminy e.Paroxysmal supraventricular tachycardia 2.What does the following rhythm strip show? a.Failure to sense b.Failure to pace c.Normal pacemaker function with a ventricular premature beat d.Failure to sense and pace 3.What is shown in the following rhythm strip? Practical Pacemaker Codes Code What is it Who gets it AOO Atrial pace, no sense, no inhibitions Sick sinus syndrome with intact conduction in the operating room with bovie. eg. Cardiac case, in OR, with bovie, with heart rate low from narcotics. AAI Atrial pace, atrial sense, inhibited by atrium Sick sinus syndrome with intact conduction system. VOO Ventricular pace, no sense, no inhibit Third degree heart block in OR with atrial fibrillation. Why atrial fibrillation? Because you cant effectively pace the atrium if it is fibrillating. VVI Ventricular pace, ventricular sense, ventricular inhibit Third degree heart block with atrial fibrillation. DOO Dual pace, no sense, no inhibitions Third degree heart block in OR with bovie. DVI Dual pace, ventricular sense, ventricular inhibit Third degree heart block with supraventricular tachycardias DDD Dual pace, dual sense, dual inhibit Third degree heart block. ICD codes I. ICDs have 4 main functions. I. They sense atrial or ventricular electrical activity, II. classify these signals to various programmed “heart rate zones,” III. deliver tiered therapies to terminate ventricular tachycardia or fibrillation, IV. pace for bradycardia. II. The most important aspect of ICD management preoperatively is deactivating the tachycardia response of the device to avoid inappropriate pacing or shocks due to electromagnetic interference. Position I Position II Position III Position IV (or use pacemaker code) Shock Chamber Antitachycar Tachycardia dia Pacing Detection Chambers Antibradyca rdia Pacing Chambers O=none O=none E=Electri O=none cal A=Atrial A=Atrial H=Hemo A=Atrial dyn. V=Ventri V=Ventri cle cle D=Dual D=Dual V=Ventri cle D=Dual Why do we care? 1) Will it quit during surgery? 2) Will the patient die if it quits? 3) Will the pacemaker cause a dysrhythmia? 4) Will the patient get shocked if he / she has an ICD? 5) Will the ICD cause a dysrhythmia? What we need to know A. B. C. D. E. F. What is the device? What brand and model? Who controls it? Does your hospital have a programmer for this make and model? What is the magnet mode? Why does the patient have a pacemaker? This is important because if the patient has it for third degree block, you should treat the device as if the his life depends on it. G. What rhythm does the patient have when the pacemaker is shut off? If the rhythm is asystole, treat the device with a certain respect and caution. Clinical Vignette #1: A 56-year-old male is admitted after a motor vehicle accident. He was intubated in the field, and is coming directly to the operating room for free air in the abdomen. A chest x-ray taken in the emergency department shows the following: A chest x-ray can be extremely informative for patients coming for emergency surgery. A chest x-ray can identify the device type, leads, and manufacturer. From this x-ray, it is clear that the patient has 3 leads: a right atrial lead, a right ventricular lead, and a coronary sinus lead. In addition, the right ventricular lead is a shocking coil, which is identified by the thicker, denser distal portion of the lead. From this chest x-ray, it is clear that the patient has an ICD due to the shocking coil, and the coronary sinus lead suggests resychronization therapy for low ejection fraction. From this x-ray, this patient should be treated like any patient with cardiomyopathy. What we need to know A. What is the device: What we need to know B. What is the model Patients carry a card you can get an X-ray of the device and see the make on the circuit board. Moreover, you can see the leads and tell what the device is. The easiest way to find out what the device is, is to call Medtronic Bradyarrhythmia Products at 800-505-4636. The phone number is on the web at http://www.medtronic.com. They are open 24/7/365. If you call and identify the patient, they will tell you if the patient has a Medtronic device, or give you a number for the other manufacturers such as Guidant, etc. What we need to know What is the magnet mode: The magnet is NOT MAGICAL. The magnet simply throws a programmable switch in the pacemaker. It changes it from one mode to another. The magnet mode depends on the brand of pacemaker, the type of device, whether magnet mode is turned on, and it may even depend on the level of battery charge in the device. In some implantable devices the magnet mode may shut off the device. In others it may make the device listen for information from a radio frequency controller. It may change modes to another type of function. The magnet throws a programmable switch. It may or may not be What we need to know What is the magnet mode cont. I. II. I. II. III. IV. III. Activates magnetic reed switch Model dependent behavior with magnet Asynchronous pacing – most common No apparent rate or rhythm change Brief asynchronous pacing and then return to programmed value Continuous or transient loss of pacing Magnets will cause most DDDs to convert to DOO at about 85 with a BOL (beginning of life) battery, and to VOO at a rate of about 65 at ERI (effective replacement interval) is reached to conserve power. What we need to know Magnet problems I. Many ICDs have built-in VOO pacemakers. If the patient has no pacemaker implanted, the built-in ICD pacemaker will become active in the event that a shock is delivered and the patient goes into asystole. Rate is usually minimal, at about 40 or so. II. If the patient’s underlying rhythm is very close to the pacemaker’s setting. I. If you reprogram the pacemaker to an DOO or VOO mode, you might cause a pacemaker stimulus at the wrong point on the patient’s cycle and induce VT or VF. III. If there is an ICD. All ICDs are inhibited by the magnet but no pacemakers with ICDs are affected by the magnet. I. In general, magnets will not affect ICD antibradycardia pacing mode or rate (except ELA and Guardian 4202/4203) Intermedics devices transiently change the pacing rate (VVI mode) to reflect battery voltage. Interrogating the device and calling the manufacturer remain the most reliable method for determining magnet response. Magnet Mode Explanation Designation Pacemaker Company AUTO Biotronik (except INOS and DROMOS) ASYNCH SYNCH Boston Scientific/Guidant Medical/CPI ASYNCH OFF EGM mode No change, magnet is ignored. OFF is the magnet mode after a “power on reset,” which can occur secondary to EMI No change in pacing. Magnet application initiates data collection Asynchronous pacing at 85 beats/min if okay, SSI at 65 beats/min regardless of original programming if ERI detected (single-step change). Most Medtronic pacemakers emit one or more ventricular pulses during the first 3-7 asynchronous events (which might be at a rate of 100 beats/min) at a reduced pulse width or voltage to demonstrate the adequacy of ventricular pacing output. Also, Medtronic pacemakers default to SSI pacing at 65 beats/min, without rate responsiveness, on detection of ERI, regardless of whether a magnet is present Medtronic St. Jude Medical (not including Telectronics) If battery okay, 10 asynchronous events at 90 beats/min, then returns to original programmed mode, without rate responsiveness. Pacing is at the lowest available rate (LRL, sleep rate, or hysteresis rate). If battery at ERI, 10 asynchronous events at 80 beats/min in the VOO mode, then either VDD (dual-chamber) or VVI (single-chamber) pacing at 11% lower than the lowest available rate. For any dual-chamber mode (DDD, DDI, or VDD), the AV delay shortens to 100 msec while the magnet is in place Asynchronous pacing at 90 beats/min if battery okay. At ERI, 80 beats/min (single-step change) in the VOO mode regardless of original programming. For any dual-chamber mode (DDD, DDI, or VDD), the AV delay shortens to 100 msec while the magnet is in place If battery okay, pacing in original programmed mode, without rate responsiveness. Pacing is at the lowest available rate (LRL, sleep rate, or hysteresis rate). If battery at ERI, either VDD (dual-chamber) or VVI (single-chamber) pacing at 11% lower than the lowest available rate. For any dual-chamber mode (DDD, DDI, or VDD), the AV delay shortens to 100 msec while the magnet is in place Asynchronous pacing at 100 beats/min if okay, 85 beats/min at ERI (single-step change). The Insignia model has an intermediate step at 90 beats/min at IFI. For Triumph and Prelude models, see Medtronic pacemakers, below Battery Test OFF “SJM” Event x-ray snapshots logo Event snapshots + Battery Test Battery Test Paces OFF etter x-ray VARIO mode logo (present in (ψ) some models) Asynchronous pacing at 98.6 beats/min gradually decreasing to <86.3 beats/min at ERI No magnet response No change in pacing. Magnet application causes pacemaker to collect data. Identity and Entity models lack this feature For a magnet placed 2 seconds, pacing mode and rate are unchanged and the device stores an EGM. If the magnet is placed ≥5 seconds, the Battery Test mode (see above) is activated. Identity and Entity models lack this feature Asynchronous pacing, with rate depending on the specific model. In general, a pacing rate of less than 90 beats/min should prompt further evaluation No magnet response VARIO results in a series of 32 asynchronous pacing events. The rate of the first 16 paces reflects battery voltage, which gradually declines from 100 to 85 beats/min at ERI. The next 15 paces are used to document the ventricular pacing capture safety margin. The rate will be 119 beats/min with gradually declining pacing voltage. The 16th pace of this group is at no output. The next pace restarts the 32-event sequence. The 32-event sequence repeats as long as the magnet remains in place Device and Electromagnetic Interference I. The most common issue arising in the perioperative I. Any apparatus that emits radiofrequency waves between 0 and 10 9 Hz can generate EMI and therefore interfere with proper device function. II. Higher frequency waves (e.g. X-rays, γ-rays, infrared, and ultraviolet light) are unlikely to cause interference with CIED function, though repeated and/or prolonged exposure to certain types of radiation can cause deterioration of insulation within the device with resultant short-circuiting or other electrical problems. II. For pacemakers in general, inhibition of pacing due to oversensing is the most common result of exposure to EMI, though in some cases, sudden asynchronous pacing, reversion to a programmed backup mode (often VVI or VOO mode), or both can be seen. III. With an ICD, EMI can result in inappropriate delivery of a defibrillatory shock. IV. Thus, if pacing modes appear to be changing abruptly or intermittently on ECG monitors, unrecognized EMI should be considered. V. The vast majority of devices now use bipolar leads; however, unipolar leads are still sometimes used when epicardial leads are placed (often in the paediatric population) and in adults with older devices. I. Bipolar leads minimize the physical distance over which the circuit is completed because both the anode and cathode are located very close to each other on the lead itself. (6 inch rule) II. In contrast, with unipolar leads, the lead tip acts as the cathode and the pulse generator acts as the anode to complete the circuit. Electrocautery (monopolar>>>>bipolar) Evoked potential monitors Nerve stimulators (twitch monitors) Fasciculations Shivering Large tidal volumes External defibrillation Magnetic resonance imaging Radio frequency ablation or lesioning Extracorporeal shock wave lithotripsy Electroconvulsive therapy What we need to know When to reprogram? I. Any rate-modulated pacing device II. Special pacing indication (hypertrophic obstructive cardiomyopathy, dilated cardiomyopathy, pediatric patients) III. Pacemaker-dependent patients (3rd degree heart block) IV. Major procedure in the chest or abdomen V. Rate enhancements present that should be disabled VI. Lithotripsy VII. Transurethral resection (electrolytes) VIII. Hysteroscopy (electrolytes) IX. Electroconvulsive therapy X. Succinylcholine use (K change) XI. Magnetic resonance imaging (generally contraindicated by device manufacturers but, as of 2009 MRI safe devices available) Reprogramming a PM to asynchronous pacing at a rate more rapid than the patient's underlying rate usually ensures that no oversensing during electrocaudery will take place, thus protecting the patient. Suggested algorithm for preoperative decision-making regarding a modern CIED with bipolar leads. Stone M E et al. Br. J. Anaesth. 2011;107:i16-i26 © The Author [2011]. Published by Oxford University Press on behalf of the British Journal of Anaesthesia. All rights reserved. For Permissions, please email: [email protected] ICD/Pacemaker failure I. Device failure is a rare perioperative occurrence that can result from a failure of the device to sense, a failure to pace, or damage to the generator. II. Most perioperative events that are thought to be pacemaker failures are really rate adaptive features that have not been disabled. I. For example, current pacemakers have minute ventilation sensors that increase the pacing rate for patients during exercise. EMI can change body impedance which might cause the pacemaker to pace at a fast rate since the pacemaker “sees” the EMI as increased physiologic demand. III. Electrical reset is also a very rare occurrence that can happen when electrocaudery directly contacts the cardiac device generator and results in device failure. I. Therapeutic radiation is the usual perioperative culprit, and it is rare in the setting of monopolar cautery or cardioversion. II. If electrical reset does occur, each CIED, depending on manufacturer and device, will default to a particular setting. While the default setting may not be optimal for one’s specific patient, it will function safely until the device can be interrogated to determine if it can be reprogrammed or replaced. III. Damage to the generator may also be caused by electrocautery applied to the generator; therefore, the path of EMI should be directed away from the generator to prevent current flow across the device. IV. Device leads may be damaged intraoperatively, leading to failures in sensing and/or pacing. Electrocaudery may produce enough current to flow from the generator to the pacing electrode and could possibly damage the tissue-lead interface. This acute injury may lead to loss of pacing and sensing. ICD/Pacemaker failure Undersensing: An intrinsic depolarization occurs in the atrium, but this depolarization is not sensed by the pacemaker. I. Improper lead position: Lead has to be positioned at an ideal location for optimal function, otherwise it can cause undersensing. II. Improper programming: Programming the lead sensitivity too high may cause undersensing. III. Lead maturation or dislodgement (as shown): I. Lead maturation or dislodgement cause sensing and capture malfunction due to poor signal strength. II. Atrial lead dislodgement. Loose connection at connector due to inadequate contact of the pin and connector there is intermittent undersensing and loss of capture III. Artifact due to monitor malfunction or loose limb lead connection. I. An abrupt loss of a portion of the QRS complex followed by a flat line can be observed. If R-R intervals are matched, 2 QRS complexes are missing during the pause. If it is due to a dislodged lead, a pacing artifact with no capture should be observed. ICD/Pacemaker Failure Causes cont: • Incompatible connector: Incompatible connector causes sensing and pacing malfunction. • Battery depletion: This can cause undersensing, loss of capture, or loss of output. • Magnet application: Magnet inhibits sensing of cardiac or non-cardiac electrical events by the pacemaker and it reverts to fixed-rate pacing function (DOO or VOO). Pacemaker reverts back to the programmed routine upon removal of the magnet. ICD/Pacemaker failure Undersensing causes cont. I. Electromagnetic interference (EMI): I. DC shock cause reversion to back-up mode, transient increases in capture threshold and loss of capture, and damage to the pulse generator and circuitry. II. Electrocautery can inhibit the pacing stimuli or trigger ventricular pacing due to atrial oversensing. II. Air pocket in unipolar pacers: I. In unipolar pacers, lead has a single stimulating electrode, the cathode, with the anode connected to an indifferent electrode, usually the outer surface of the pulse generator that has to be in constant contact with the subcutaneous tissue of the person. II. In dry pocket situations there is inadequate contact between pulse generator and tissue causing inappropriate sensing and pacing. III. Post defibrillation or cardioversion: DC shock causes pacemaker reversion to back-up mode, transient increases in capture threshold and loss of capture, and damage to the pulse generator and circuitry. IV. Electrolyte abnormality such as hyperkalemia or acidosis: Hyperkalemia causes both sensing and pacing malfunction due to a reduction of the electronegativity of the resting myocardial potential. I. Potassium, calcium, and magnesium abnormalities can raise depolarization thresholds. II. Potassium flux, ionized calcium levels, and acid-base equilibrium can be affected by hyperventilation and hypoventilation. ICD/Pacemaker failure Failure to capture •Dry (air) pocket in unipolar pacer •Circuit failure •Impending battery depletion •Inadequate programmed output with higher threshold •Insulation break •Partial conductor coil fracture •Lead maturation, dislodgement, or perforation •Poor or incompatible connection at connector block •Conditions that increase the capture threshold, such as metabolic and electrolyte abnormalities (Potassium), medications, and myocardial infarction ICD/Pacemaker failure Factors increasing pacemaker threshold I. Myocardial ischemia or infarct II. Electrolyte disturbance I. Potassium II. Calcium III. Acidosis or alkalosis IV. Hypoxia or hypercapnia V. Abnormal antiarrhythmic drug level especially Class I and beta blockers ICD/Pacemaker failure Electroconvulsive therapy (ECT) I. II. ECT is relatively safe for patients with pacemakers, because of the localized application of the electrical stimulus to the head, hence a low probability for the occurrence of problems. Sometimes the seizure may generate myopotentials which may inhibit the pacemaker, and transient electrocardiographic changes (e.g., increased Pwave amplitude, altered QRS shape, T-wave and ST-T abnormalities) may occur and additional cardiac complications (e.g., arrhythmia or ischemia) may occur in patients with pre-existing cardiac disease. TENS I. II. III. Electrical stimulating techniques such as transcutaneous electrical nerve stimulation (TENS) consists of several electrodes placed on the skin and connected to a pulse generator that applies 20 μs rectangular pulses of 1– 200 V and 0–60 mA at a frequency of 20–110 Hz. This repeated frequency is similar to the normal range of heart rates, so it can create a far field potential that may inhibit a cardiac pacemaker. TENS can be used safely in patients with pacemakers and defibrillators with close monitoring and use in close proximity to the device is not advised ICD/Pacemaker failure Radiofrequency ablation (RFA) I. The radiofrequency current path should be kept as far away from the pulse generator and lead system as possible and to avoid direct contact between the ablation catheter and the device Radiation therapy I. II. III. IV. V. The high-energy ionizing radiation used in radiation therapy can cause significant damage to the semiconductors of pacemakers, even at very small doses. Generally, doses in excess of 5000 rads are required to cause pacemaker malfunction but as little as 1000 rads may induce pacemaker failure or cause runaway pacemaker. Pulse generator recovery may occur long after the end of the radiation treatment, but it is mostly incomplete, and the pacemaker cannot be used reliably thereafter. Thus, in pulse generators exposed to radiation, transient loss of function should be regarded as a precursor of permanent damage. Hence it is essential to follow guidelines for ensuring the lowest possible radiation dose to the pacemaker and careful follow-up should be performed during and after completion of the radiation therapy. The device must be outside the field of radiation. The low-energy X-rays used for diagnostic radiology have not been reported to have any adverse effect in pacemakers. Pacemaker failure BP stable Observe O2 Atropine Hypotension Epinephrine Isoprotenerol Asystole Temporary pacing CPR Summary Pacemaker and AICD implanted patients undergoing emergency surgery: 1. Try get information regarding pacemaker or AICD at earliest possible or if time permits 2. Contact pacemaker or AICD clinic or manufacturer 3. Reprogram the device function in selective group of patients 4. If possible avoid electrocautery use within 6 inches of the device 1. If necessary consider use of bipolar or harmonic scalpel 2. Be ready for alternate mode of pacemaker and defibrillator if necessity arises References 1. 2. 3. 4. 5. 6. M. E. Stone, B. Salter,and A. Fischer Perioperative management of patients with cardiac implantable electronic devices Br. J. Anaesth. (2011) 107 Miller: Miller's Anesthesia, 7th ed. Chapt 43. Apfelbaum JL, Belott P, Connis RT, et al.; for the American Society of Anesthesiologists Committee on Standards and Practice Parameters. Practice advisory for the perioperative management of patients with cardiac implantable electronic devices: pacemakers and implantable cardioverter-defibrillators. Anesthesiology 2011;114:247-61. American Society of Anaesthesiologists Task Force on Perioperative Management of Patients with Cardiac Rhythm Management Devices. Practice advisory for the perioperative management of patients with cardiac rhythm management devices: pacemakers and implantable cardioverter-defibrillators: a report by the American Society of Anaesthesiologists Task Force on Perioperative Management of Patients with Cardiac Rhythm Management Devices. Anesthesiol 2005;103:186-98. Managing Cardiovascular Implantable Electronic Devices (CIEDs) During Perioperative Care Jacques P. Neelankavil, MD; Annemarie Thompson, MD; Aman Mahajan, MD, PhD. APSF Newsletter, Fall 2013. M.L. Dohrmann, N.F. GoldschlagerMyocardial stimulation threshold in patients with cardiac pacemakers: effect of physiologic variables, pharmacologic agents, and lead electrodes Cardiol Clin, 3 (1985), pp. 527–537