* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Unit 3 Quantum Numbers PPT
Quantum computing wikipedia , lookup
Ferromagnetism wikipedia , lookup
X-ray fluorescence wikipedia , lookup
Relativistic quantum mechanics wikipedia , lookup
Chemical bond wikipedia , lookup
Quantum teleportation wikipedia , lookup
Interpretations of quantum mechanics wikipedia , lookup
Orchestrated objective reduction wikipedia , lookup
Quantum machine learning wikipedia , lookup
Renormalization wikipedia , lookup
Quantum key distribution wikipedia , lookup
Symmetry in quantum mechanics wikipedia , lookup
Canonical quantization wikipedia , lookup
Quantum group wikipedia , lookup
Wave–particle duality wikipedia , lookup
Tight binding wikipedia , lookup
Hidden variable theory wikipedia , lookup
Molecular orbital wikipedia , lookup
Particle in a box wikipedia , lookup
Quantum state wikipedia , lookup
History of quantum field theory wikipedia , lookup
EPR paradox wikipedia , lookup
Theoretical and experimental justification for the Schrödinger equation wikipedia , lookup
X-ray photoelectron spectroscopy wikipedia , lookup
Atomic theory wikipedia , lookup
Auger electron spectroscopy wikipedia , lookup
Quantum electrodynamics wikipedia , lookup
Electron-beam lithography wikipedia , lookup
Hydrogen atom wikipedia , lookup
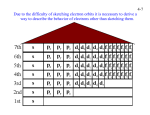
Light and Energy In 1900 Max Planck helped us move toward a better understanding of electromagnetic radiation. Matter can gain or lose energy only in small, specific amounts called quanta. A quantum is the minimum amount of energy that can be gained or lost by an atom. Atomic Emission Spectrum Electrons gain energy and ‘jump’ to a higher energy level. This is an unstable excited state. The electrons rele se energy when they return to their ground state. We see this energy as light. The Bohr Quantum Model The electron in a hydrogen atom moves around the nucleus only in certain allowed circular orbits Ground State: The lowest possible energy level. Excited State: Higher energy level. Atomic Emission Spectra Wave-Particle Duality Theory (Einstein) Is energy a wave like light, or a particle? BOTH Atomic Theory Louis de Broglie (1892 – 1987) •Matter has characteristics of both waves and particles •Electrons move in wavelike motion in the •circular orbits Heisenberg Uncertainty Principle There is a fundamental limitation to just how precisely we can know both the position and momentum of a particle at a given time Schrodinger Came up with an equation to describe the probability of finding an electron within a 3D area of space. These equations are called wavefunctions. The wavefunctions describe orbitals. Orbitals are the building block of electron arrangement. Quantum Numbers At the conclusion of our time together, you should be able to: List and define each of the 4 quantum numbers. Relate these numbers to the state, city, street and home address for the electron. Give the maximum number of electrons for each level and sublevel. Draw the basic shape of the 4 sublevels. Quantum Numbers The location of an electron is described with 4 terms or the 4 quantum numbers. - Energy Level = n - Sublevel = l - Orbital = ml - Spin = ms Principal Quantum Number - n Symbol = n Represents the main energy level of the electron and its distance from the nucleus Equation: 2n2 - shows how many electrons can be in each energy level (e.g. 3rd energy level: 2(3)2 = 18 total possible ein this energy level) Your turn: How many electrons in the 4th energy level? 32 Principal Quantum Number - n In an address analogy, this would be the state in which the electron would probably be found. Presently, we can find electrons in 7 states. Values are 1-7 The Second Quantum Number - l This number describes sublevels, shapes of these sublevels and is the Angular momentum quantum number. The first 4 sublevels are named s, p, d, f. Each sublevel has a unique number of orbitals and shape: Shape of the “s” Sublevel • s for "Sphere": the simplest shape, or shape of the simplest atoms like hydrogen and helium • Contains 1 orbital. Shapes of the “p” Sublevel • p for "Peanut/Petal": a more complex shape that occurs at energy levels 2 and above • Contains 3 orbitals. Shapes of the “d” Sublevel • d for "Double Peanut/Petal": a complex shape occurring at energy levels 3 and above • Contains 5 orbitals. Shapes of the “f” Sublevel • f for "Flower": bizarre-shaped orbitals for electrons of very large atoms • Contains 7 orbitals. The Second Quantum Number - l In the address analogy, this would be the city in which the electron would probably be found. In the first state there would be 1 city, in the second state there would be 2 cities... The Second Quantum Number - l The 2nd Quantum Number = 0 to (n-1) Gives the sublevel type the electron is found in: s, p, d, or f. Therefore: For s, l = 0 For p, l = 1 For d, l = 2 For f, l = 3 n=1 n=2 n=3 n=4 l=0 l = 0, 1 l = 0, 1, 2 l = 0, 1, 2, 3 designated by letters l = 0 s orbital l = 1 p orbital l = 2 d orbital l = 3 f orbital s p n=1 n=2 n=3 n=4 n=5 n=6 n=7 d f The Third Quantum Number - m This number describes the specific orbital that an electron is in on a specific sublevel. Value = –l to 0 to +l s has one orbital, p has three orbitals, d has five orbitals and f has 7 orbitals. The Third Quantum Number - m For s, l = 0; For p, l = 1; For d, l = 2; For f, l = 3 Values for m: s = ___ p = ___ ___ ___ 0 -1 0 +1 d = ___ ___ ___ ___ ___ -2 -1 0 1 2 f = ___ ___ ___ ___ ___ ___ ___ -3 -2 -1 0 1 2 3 The Fourth Quantum Number - s This number describes the spin of the electrons in an orbital. There can be two electrons in each orbital as long as they are spinning in opposite directions. In the address analogy, this would be the house number of the electron. There can only be 2 houses on each street Values are +1/2 = clockwise spin -1/2 = counter clockwise spin Values for s: s = (-1/2, +1/2) Each orbital can hold only two oppositely spinning electrons, so there are only two possible “s” values +1/2 -1/2 Quantum Numbers Let’s see if you can: List and define each of the 4 quantum numbers. Relate these numbers to the state, city, street and home address for the electron. Give the maximum number of electrons for each level and sublevel. Draw the basic shape of the 4 sublevels. 1 s orbital 3 p orbitals 5 d orbitals each orbital holds 2e-4th quantum numberms spin 7 f orbitals s p n=1 n=2 n=3 n=4 n=5 n=6 n=7 d f 5p 4d What are the quantum numbers for this electron? n= 5s 4p 3d 4s 4 3p L= 1 3s 2p m = +1 s= -1/2 2s Usually quantum numbers are expressed like this: 1s (4, 1, +1, -1/2) 5p 4d What are the quantum numbers for this electron? 5s 4p 3d 4s n= L= 3 2 m = -1 s = +1/2 3p 3s 2p 2s 1s (3, 2, -1, +1/2) 5p 4d 5s Which electron has this set of quantum numbers? (2, 0, 0, -1/2) 4p 4s 3p 3s 2p 2s 1s 3d 5p 4d 5s Which electron has this set of quantum numbers? (3, 1, -1, -1/2) 4p 4s 3p 3s 2p 2s 1s 3d How do we know when an electron has moved from an excited state to the ground state? The electron will 1. 2. 3. 4. 5. Release a photon. Release a specific amount of energy. Release a specific color. Release a quantum of energy. All of the above are correct. What does the second quantum number (l) describe? 1. 2. 3. 4. Orbital shape. Energy level. Electron spin. Orbital orientation. Which quantum number has values of +½ or –½? 1. 2. 3. 4. Orbital shape. Energy level. Electron spin. Orbital orientation. Which of the following is not a possible orbital shape? 1. 2. 3. 4. s p z f What is currently the highest possible principal quantum number an electron can have? 1. 2. 3. 4. No limit 5 6 7 How many electrons will the 4th energy level hold? 1. 2. 3. 4. 5. No limit 8 18 32 50 The p sublevel would look like a 1. 2. 3. 4. 5. sphere petal double petal flower mess Quantum Numbers At the conclusion of our time together, you should be able to: Continue to relate Quantum Numbers to the state, city, street and home address for the electron. Give the 4 quantum number for every electron of every atom on the periodic table. Let’s Try Helium H is 1s1 He has 2 electrons, can we add another electron spinning in the other direction in the first energy level of the s sublevel with its 1 spherical orbital? Yes, He is 1s2 He has 2 electrons spinning in opposite directions in the s sublevel with its spherical shape with 1 orientation. Remember the Quantum # Summary for Hydrogen 1s1 Hydrogen has one electron spinning in a clockwise direction in the first energy level that has one spherical orbital in its s sublevel. The 4 quantum numbers for H are: 1, 0, 0, +1/2 The Pauli Exclusion Principle No two electrons in an atom can have the same set of four quantum numbers. Therefore, the second electron that He has must have a different set of 4 Quantum Numbers. What would they be?? The 4 Quantum Numbers of Helium n 1 l 0 m 0 s -1/2 The 4 Quantum Numbers of Helium No two electrons in an atom can have the same set of four quantum numbers. What principle?? Pauli Exclusion Principle Therefore, the second electron that He has must have a different set of 4 Quantum Numbers. What would they be?? 1, 0, 0, -1/2 Let’s Try Lithium He is 1s2 He has 2 electrons, can we add another electron spinning in another direction in the first energy level of the s sublevel with its 1 spherical orbital? No, the third electron must go to the 2nd energy level which has 2 sublevels, s and p, s with its one spherical orbital and p with its 3 orientations of its petal shaped orbitals. Let’s Try Lithium So the address for all 3 electrons of Li is: 1s2 2s1 This is called the electron configuration for Li and basically says that the first two electrons of Li are in the s sublevel with its one spherical shape with the 3rd electron in the 2 energy level, s sublevel with its bigger spherical shape. The Quantum #’s of the 3rd electron: The 4 Quantum Numbers for the 3rd Electron of Lithium n 2 l 0 m 0 s +1/2 Now Beryllium: Add one more electron to Li : 1s2 2s1? Where would it go?? 1s2 2s2 The 2s sublevel with its one spherical orbital can hold 2 electrons spinning in opposite directions. The Quantum #’s of the 4th electron: The 4 Quantum Numbers for the 4th Electron of Beryllium n 2 l 0 m 0 s -1/2 What About Boron: Add one more electron to Be: 1s2 2s2? Where would it go?? 1s2 2s3 ? No 1s2 2s2 2p1 The 2s sublevel with its one spherical orbital is full. We must now start filling the 2p sublevel with its 3 orbitals The Quantum #’s of the 5th electron: The Electrons of the 2nd Energy Level for Boron s = ___ 0 p = ___ ___ ___ -1 0 +1 The 4 Quantum Numbers for the 5th Electron of Boron n 2 l 1 m -1 s +1/2 Let’s Move to Carbon: Add one more electron to B: 1s2 2s2 2p2 The 2p sublevel has 3 orbitals. However, we can’t put another electron in the first orbital of p. Why? Hund’s Rule: Orbitals of equal energy are each occupied by one electron before any orbital is occupied by a second electron. The Electrons of the 2nd Energy Level for Carbon s = ___ 0 p = ___ ___ ___ -1 0 +1 The 4 Quantum Numbers for the 6th Electron of Carbon n 2 l 1 m 0 s +1/2 Nitrogen: Add one more electron to C: 1s2 2s2 2p3 The 3rd electron in the p sublevel must go into the 3rd orbital. Why? Again Hund’s Rule: Orbitals of equal energy are each occupied by one electron before any orbital is occupied by a second electron. The Electrons of the 2nd Energy Level for Carbon s = ___ 0 p = ___ ___ ___ -1 0 +1 The 4 Quantum Numbers for the 7th Electron of Nitrogen n 2 l 1 m 1 s +1/2 Oxygen: Add one more electron to N: 1s2 2s2 2p4 The 4th electron in the p sublevel must begin to double up the electrons of the three p orbitals. The Electrons of the 2nd Energy Level for Carbon s = ___ 0 p = ___ ___ ___ -1 0 +1 The 4 Quantum Numbers for the 8th Electron of Oxygen n 2 l 1 m -1 s -1/2 Fluorine: Add one more electron to O: 1s2 2s2 2p5 Where would the 5th electron for the 2p orbitals go? The Electrons of the 2nd Energy Level for Fluorine s = ___ 0 p = ___ ___ ___ -1 0 +1 The 4 Quantum Numbers for the 9th Electron of Fluorine n 2 l 1 m 0 s -1/2 Neon: Add one more electron to F: 1s2 2s2 2p6 Where would the 6th electron for the 2p orbitals go? The Electrons of the 2nd Energy Level for Neon s = ___ 0 p = ___ ___ ___ -1 0 +1 The 4 Quantum Numbers for the 10th Electron of Neon n 2 l 1 m 1 s -1/2 What About Sodium: Add one more electron to Ne: 1s2 2s2 2p7 No!! The 2p sublevel with its 3 orbitals is full. We must now go to the 3rd energy level with its 3 sublevels, s, p, and d, and start filling electrons all over again! The Electrons of the 3rd Energy Level for Sodium s = ___ 0 p = ___ ___ ___ -1 0 +1 d = ___ ___ ___ ___ ___ -2 -1 0 1 2 The 4 Quantum Numbers for the 11th Electron of Sodium n 3 l 0 m 0 s +1/2 What About Magnesium: Add one more electron to Na: 1s2 2s2 2p6 3s2 Remember, the s sublevel with its one spherical orbital can have one more electron. The Electrons of the 3rd Energy Level for Magnesium s = ___ 0 p = ___ ___ ___ -1 0 +1 d = ___ ___ ___ ___ ___ -2 -1 0 1 2 The 4 Quantum Numbers for the 12th Electron of Magnesium n 3 l 0 m 0 s -1/2 Aluminum: Add one more electron to Mg: 1s2 2s2 2p6 3s2 3p1 Remember, the s sublevel with its one spherical orbital can only have 2 electrons. The next electron must start to fill the 3p sublevel with its 3 orbitals. The Electrons of the 3rd Energy Level for Aluminum s = ___ 0 p = ___ ___ ___ -1 0 +1 d = ___ ___ ___ ___ ___ -2 -1 0 1 2 The 4 Quantum Numbers for the 13th Electron of Aluminum n 3 l 1 m -1 s +1/2 Silicon to Argon: Keep adding one more electron to Al: 1s2 2s2 2p6 3s2 3p2-6 Remember Hund’s rule. We will put one electron in each of the p orbitals and then come back to double up. The Electrons of the 3rd Energy Level for Silicon to Argon s = ___ 0 p = ___ ___ ___ -1 0 +1 d = ___ ___ ___ ___ ___ -2 -1 0 1 2 The 4 Quantum Numbers for the 18th Electron of Argon n 3 l 1 m 1 s -1/2 Quantum Numbers Let’s see if you can: Continue to relate Quantum Numbers to the state, city, street and home address for the electron. Give the 4 quantum number for every electron of every atom on the periodic table. The 5f sublevel with its orbitals would look like a 1. 2. 3. 4. 5. sphere petal double petal flower mess How many orbitals are in the 5f sublevel? 1. 2. 3. 4. 5. No limit 1 3 5 7 How many electrons will the 4f sublevel hold? 1. 2. 3. 4. 5. 2 6 10 14 18 In the address analogy, the first quantum number is the state and the second quantum number is the city. How many cities are there in the second state? 1. 2. 3. 4. 5. 1 2 3 4 5 In the address analogy, the second state can have how many total streets? 1. 2. 3. 4. 5. 1 2 3 4 5 Quantum Numbers At the conclusion of our time together, you should be able to: Explain the Aufbau principle and the diagonal rule. Use Hund’s rule in an orbital filling diagram. Give the quantum numbers for every electron of every atom on the periodic table. Draw the electron configuration and orbital notation for every element. Now Potassium: We should now start to fill the 3d sublevel with its 5 orbitals. 1s2 2s2 2p6 3s2 3p6 3d1 But now we must consider ??? the Aufbau Principle: An electron will occupy the lowest energy level orbital that can receive it. Look at the energy needed for 3d vs. 4s: The Aufbau Principle You will need to remember this chart so that you place electrons in the correct order. An easy way to remember the filling order is to follow the diagonal rule. Check it out on the next slide: Maybe You’ve Seen This Chart??? 1s 2s 2p 3s 3p 3d 4s 4p 4d 4f 5s 5p 5d 5f 5g? 6s 6p 6d 6f 6g? 6h? 7s 7p 7d 7f 7g? 7h? 7i? It Represents the Diagonal Rule Steps: 1. Write the energy levels top to bottom. 2. Write the orbitals in s, p, d, f order. Write the same number of orbitals as the energy level. 3. Draw diagonal lines from the top right to the bottom left. 4. To get the correct order, follow the arrows! Diagonal Rule 1s 2s 2p 3s 3p 3d 4s 4p 4d 4f 5s 5p 5d 5f 5g? 6s 6p 6d 6f 6g? 6h? 7s 7p 7d 7f 7g? 7h? By this point, we are past the current periodic table so we can stop. 7i? The Aufbau Principle But there is even an easier way to remember the filling order. Have you noticed areas in the periodic table where certain sublevels are filling?? Check it out on the next slide: The 4 Blocks of the Periodic Table The Aufbau Principle So, what block is always filling on the left side of the periodic table?? The s block!! What period is it?? The 4th!! Therefore, 4s will begin filling before we fill 3d!!! So is Potassium? 1s2 2s2 2p6 3s2 3p6 3d1 No!!! 1s2 2s2 2p6 3s2 3p6 4s1 The Electrons of the 4rd Energy Level for Potassium and Calcium s = ___ 0 p = ___ ___ ___ -1 0 +1 d = ___ ___ ___ ___ ___ -2 -1 0 1 2 The 4 Quantum Numbers for the 20th Electron of Calcium n 4 l 0 m 0 s -1/2 The “D” Block Electrons The next electron for Scandium will now start to fill the “D” block. Please note that the energy level for the d sublevel is not 4. We have not filled 3d yet. Therefore, the “D” block will always be one energy level behind the current energy level. Remember the Shape of the “d” Orbitals • d for "Double Peanut/Petal": complex shape occurring at energy levels 3 and above •How many orbitals (orientations) does “d” have? •5 So for Scandium to Zinc Following Hund’s Rule, we will put one electron in each of the 3d orbitals before we begin to double up the electrons in each orbital. Remember that the “d” block is always one energy level behind. The Electrons of the 3rd Energy Level for Scandium to Zinc s = ___ 0 p = ___ ___ ___ -1 0 +1 d = ___ ___ ___ ___ ___ -2 -1 0 1 2 The 4 Quantum Numbers for the Circled Electron of Zinc n 3 l 2 m 1 s +1/2 Gallium to Krypton So, what block is always filling on the right side of the periodic table?? The p block!! What period is it?? Back to the 4th!! Therefore, 4p will begin filling after we fill 3d!!! The Electrons of the 4rd Energy Level for Gallium to Krypton s = ___ 0 p = ___ ___ ___ -1 0 +1 d = ___ ___ ___ ___ ___ -2 -1 0 1 2 The 4 Quantum Numbers for the Circled Electron of Krypton n 4 l 1 m -1 s -1/2 Rubidium and Strontium So, what block is always filling on the left side of the periodic table?? The s block!! What period is it?? 5th!! Remember, the 4th energy level has 4 sublevels, s, p, d, and f. Because of the Aufbau Principle, d and f will fill later. Rubidium and Strontium Let’s do the electron configuration for these two elements 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s1 or 5s2 How About Yttrium to Cadmium? 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d1-10 Indium to Xenon? 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p1-6 Cesium and Barium? 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s1-2 do the 4 quantum numbers for 55th electron of Barium. Now The 4 Quantum Numbers for the Circled Electron (55th) of Barium n 6 l 0 m 0 s +1/2 The “F” Block Elements Now we come to a confusing part of the periodic table. Note that element #57, Lanthanum, is in the “d” block but the next element #58, Cerium, drops down to the “f” block. The Periodic Table The “F” Block Elements Also note that according to the Aufbau Principle, after 6s should fill 4f and then 5d. The “F” Block Elements Recent discoveries suggest that Lanthanum is not the first element of the 4f block as previously thought, but really is the first element of the 5d block. From there we will move to the 4f block. Lanthanum? 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 5d1 Then Cerium is 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 5d1 4f1 The Rest of the 4f Block #58 Praseodymium to #71 Lutetium 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 5d1 4f1-14 The Electrons of the 4f Sublevel 6s = ___ 0 6p = ___ ___ ___ -1 0 +1 5d = ___ ___ ___ ___ ___ -2 -1 0 1 2 4f = ___ ___ ___ ___ ___ ___ ___ -3 -2 -1 0 1 2 3 The 4 Quantum Numbers for the Circled Electron (67th) of Lutetium n 4 l 3 m -1 s -1/2 Now Back to the “D” Block Elements As we follow the numbers on the Periodic Table, you will see that element #72, Hafnium, is back in the “d” block. Therefore, Hafnium’s configuration would be: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 5d1 4f14 5d2 Right?? Wrong!! Now Back to the “D” Block Elements Why? Count the total electrons… 73 not 72 Why? Because 5d2 includes 5d1 I’ve counted 5d1 twice!! Therefore, I must do this 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 5d1 4f14 5d2 The Rest of the “D” Block Elements Tantalum to Mercury 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 5d1 4f14 5d3-10 Now to the 6p Block Elements Thallium to Radon 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 5d1 4f14 5d10 6p1-6 What’s Next #87 - #88, Francium and Radium 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 5d1 4f14 5d10 6p1-6 7s1-2 Now We Run into the Crazy Area #89, Actinium 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 5d1 4f14 5d10 6p1-6 7s2 6d1 #90 - #103, Thorium to Lawrencium 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 5d1 4f14 5d10 6p1-6 7s2 6d1 5f1-14 Finally, We Finish Up in the D Block #104 - #112, Rutherfordium to Copernicium Don’t forget to cross out the 6d1 electron when you come back to the “d” block 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 5d1 4f14 5d10 6p1-6 7s2 6d1 5f14 6d2-10 Quantum Numbers Let’s see if you can: List and define the 4 principles that are part of quantum numbers. List and define each of the 4 quantum numbers. Give the quantum number for every electron of every atom on the periodic table. Your Turn Let’s see if you can do the last 4 orbital filling diagrams and the 4 quantum numbers for the last electron of #112, Copernicium 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 5d1 4f14 5d10 6p1-6 7s2 6d1 5f14 6d10 The Electrons of the 4 Configurations for Copernicium 7s = ___ 0 6p = ___ ___ ___ -1 0 +1 6d = ___ ___ ___ ___ ___ -2 -1 0 1 2 5f = ___ ___ ___ ___ ___ ___ ___ -3 -2 -1 0 1 2 3 The 4 Quantum Numbers for the Circled Electron (112th) of Copernicium n 6 l 2 m 2 s -1/2 It is impossible to determine both the position and the momentum of an electron at the same time. An electron occupies the lowest energy level available. No two electrons in the same atom can have the same set of four quantum numbers. In other words, no two electrons can be in the same place at the same time. Orbitals of equal energy are each occupied by ONE electron before any orbital is occupied by a SECOND electron All electrons in a single occupied orbital must have the same spin. Symbol = n Represents the main energy level of the electron Range = 1- 7 Ex. = 3s2 Principal Quantum number = 3 Symbol = l (small letter L) Represents the shape of the orbital (also called sublevel) Range = 0 – n-1 (whole number) Shapes: 0 = s (sphere) 1 = p (petal) 2 = d (double petal) 3 = f (flower) Ex. = 3s2 AM Quantum number = 0 Symbol = m Represents the orientation of the orbital around the nucleus Each line holds 2 electrons m = -l to +l; Therefore: s = 0, p = 3, d = 5… ___ = s 0 ___ ___ ___ = p -1 0 +1 ___ ___ ___ ___ ___ = d -2 -1 0 +1 +2 ___ ___ ___ ___ ___ ___ ___ = f -3 -2 -1 0 +1 +2 +3 Ex. = 3s2 Magnetic Quantum number = 0 2 Spin States Clockwise spin = +1/2 (upward arrow) Counterclockwise spin = -1/2 (downward arrow) A single orbital can hold two electrons, but they must have opposite spins Ex. = 3s2 Spin Quantum number = -1/2 Congratulations!!!! You can now do the complete electron configurations, orbital notations and give 4 quantum numbers (addresses) for every electron of every element on the periodic table!!! GRADING SCALE • A = upright and taking air • B = eyes notable open • C = responding to the environment • D = comatose • F = decomposed