* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Chemical Bonding
X-ray photoelectron spectroscopy wikipedia , lookup
Molecular orbital wikipedia , lookup
Atomic orbital wikipedia , lookup
Surface properties of transition metal oxides wikipedia , lookup
Physical organic chemistry wikipedia , lookup
State of matter wikipedia , lookup
Metastable inner-shell molecular state wikipedia , lookup
Electrochemistry wikipedia , lookup
Rutherford backscattering spectrometry wikipedia , lookup
Ionic liquid wikipedia , lookup
Aromaticity wikipedia , lookup
Nanofluidic circuitry wikipedia , lookup
Electron configuration wikipedia , lookup
Homoaromaticity wikipedia , lookup
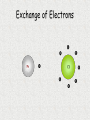
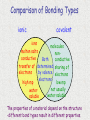
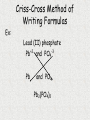
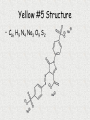
Unit 3: Chemical Formulas and Bonding Electron Dot diagrams • Electron dot diagrams show the valence electrons around an atom. In most molecules and compounds a complete octet is achieved for each atom: Al N • Most monatomic ions have an electron configuration of noble gases: 7 valence e-s F + e- F 8 valence e-s Drawing Lewis Dot Structures • To visualize valence e-, we will use Lewis Dot Structures. • Step 1: The element symbol represents the nucleus and all e- except valence. • Step 2: From the periodic table, determine the number of valence e-. • Step 3: Each “side” of symbol represents an orbital. Draw two dots on one side, then one for each of the remaining three sides. Additional electrons should then be paired. Lewis Dot Structures • Ex: carbon step 1: C step 2: 4 valence e-s step 3: C Lewis Dot Structures • Ex: bromine step 1: Br step 2: 7 valence e-s step 3: Br Chemical Bonding What holds things together? Let’s examine the melting point of compounds across two periods. What is the trend? Conductivity - high Conductivity - low Nonconductive Chlorides of Period 2 compound LiCl BeCl2 BCl3 CCl4 NCl3 OCl2 melting point 610 415 -107 -23 -40 Cl2 -121 -102 NaCl MgCl2 AlCl3 SiCl4 PCl3 SCl6 Cl2 801 -51 -102 Chlorides of Period 3 compound melting point 714 high 193 -69 -112 low Bonding How can we explain the melting point behavior across a period? Bonding between atoms changes across a period… • Bonding involves the valence electrons or outermost shell (or highest shell) electrons • Atoms form bonds to become more stable – electrons are gained, lost or shared to achieve stability. • The properties of a compound are different from the properties of the atoms that make up the compound. Ex: NaCl Types of Bonds 1. Ionic bond Transfer of e- from a metal to a nonmetal and the resulting electrostatic force that holds them together forms an ionic compound. EX: Na+ + Cl- NaCl (neutral) Ionic Bonding Ionic bonds involve the formation of positive and negative ions that then attract each other. Metals form positive ions by losing electrons Nonmetals form negative ions by gaining electrons Next Slide Ionic Bonding Example 1 Sodium has 1 valence electron which it needs to lose. Na Cl Chlorine has 7 valence electrons and needs to pick up 1 electron. Next Slide Ionic Bonding Example 1 The sodium loses its electron to the chlorine. +1 -1 Na Cl This makes the sodium +1 and the chlorine -1 They attract each other forming the compound NaCl Next Slide Ionic Bonding Example 2 Magnesium has 2 valence electrons which it needs to lose. O Mg Oxygen has 6 valence electrons, It needs to pick up 2 electrons. Next Slide Ionic Bonding Example 2 Magnesium loses both of its outer electrons to the oxygen. Mg Next Slide O Ionic Bonding Example 2 This gives the magnesium a +2 charge and the oxygen a -2 charge -2 +2 Mg O They join together to form the compound MgO. Next Slide Exchange of Electrons Ionic Bonding When atoms bond, the properties of the new compound are DIFFERENT from the properties of the elements that made them up. Ionic compounds have several characteristics in common due to the presence of the ionic bond. These characteristics include: Crystalline structure (the formula gives the ratio between the ions making up the substance) High melting points, making them solids at room temperature Usually water soluble (can dissolve in water) Electrolytes when in solution (conduct electricity) Ionic Bonding Sodium chloride (NaCl) is held together by an ionic bond. The properties of sodium chloride are: Sodium chloride forms a cube shaped crystalline solid. Melting point = 801˚C Boiling point = 1413˚C Highly soluble in water Strong electrolyte Cl- Na+ Na + ClNa + Cl- Na + Cl- < TARGET="display"> Types of Bonds 2. Covalent bond Formed from the sharing of e- pairs between two or more nonmetals resulting in a molecule. EX: H2 + O H2O Covalent Bonding Definition - bond formed due to the sharing of electrons between nonmetals. The high attraction for electrons of nonmetals results in the nonmetals attempting to remove electrons from each other. Since neither nonmetal is able to give up electrons they are forced to share the electrons. Covalent Bonding Example 1 Bromine and Fluorine both have 7 valence electrons and very high attraction for electrons. Br F Covalent Bonding Example 1 Since neither fluorine or bromine are able to lose electrons they get drawn together until their outer orbits overlap and one electron from each atom goes back and forth between the two atoms. Br F This creates the compound BrF. Covalent Bonding Example 2 Hydrogen and Oxygen can both pick up electrons. (If hydrogen loses its only electron it will end up as a nucleus with no electrons around it.) They will share electrons to form covalent bonds. This results in the formula H2O H O H Covalent Bonding Example 3 Seven of the elements have such high attraction for electrons that they will never exist as individual, unattached atoms. Anytime these elements are present in pure form they will bond to other atoms of the same element. For example a fluorine atom will readily bond to a second fluorine atom. Resulting in F2. F F Covalent Bonding These elements are called diatomic elements, and the molecules they form are called diatomic molecules. The definition of a diatomic molecule is: A molecule made up of 2 atoms of the same element. Covalent Bonding The seven diatomic elements are: Hydrogen, H2 Nitrogen, N2 Oxygen, O2 Fluorine, F2 Chlorine, Cl2 Bromine, Br2 Iodine, I2 Covalent Bonding Covalent compounds are made up of small units called molecules. The formula for a covalent compound tells the actual number of atoms, of each element, found in each molecule. For example: The formula for water is H2O. This formula indicates that each water molecule is made up of two Hydrogen atoms and one Oxygen atom. What is a “polar” covalent bond? • Covalent bonds involve sharing electrons. • The electrons may be shard equally (nonpolar covalent) or unequally (polar covalent). • Example: H2 shares electrons equally, but HCl does not. Therefore, H2 contains a nonpolar covalent bond and HCl contains a polar covalent bond. Polarity of water • Take for example H2O. When we draw the structure it looks like: O H H • The oxygen atom pulls electrons away from the hydrogen atoms. This unequal sharing results in polar bonds, which have a “more negative” end and a “more positive” end. Polar Water Molecule more negative EX: O more positive H H H2O is a polar molecule. Bond polarity affects the properties of a material such as melting and boiling points, crystal structure and acidity. Covalent Bonding The characteristics shared by covalent compounds are: Molecular structure –individual units Low melting and boiling points, most covalent compounds are gases or liquids at room temperature (the larger the molecule the higher its melting and boiling point) Soluble in covalent solvents such as alcohol or benzene. Nonelectrolytes gas atoms and molecules Covalent Bonding Paradichlorobenzene (moth balls) (C6H4Cl2) is a covalent compound. Its properties are: Molecular solid Cl Melting point = 53.1˚C H Boiling point = 174.55˚C Soluble in alcohol, ether, acetone and benzene Nonelectrolyte H C H C C C C C Cl H Comparison of Bonding Types ionic covalent ions molten salts conductive molecules nonconductive Both transfer of determined sharing of by valence electrons electrons electrons high mp low mp not usually water water soluble soluble The properties of a material depend on the structure -different bond types result in different properties. Polyatomic Ions/Radicals Some groups of atoms are covalently bonded together so strongly that the stay together during chemical reactions and act as a single unit. These groups of atoms become charged, with the charge being spread out through out the group. These groups are called polyatomic ions or radicals. Polyatomic Ions/Radicals Definition - groups of atoms bonded together that act as a charged unit. Examples: Ammonium - NH4+1 Sulfate - SO4-2 Acetate - C2H3O2-1 Phosphate - PO4-3 What kind of bond do you think hold the atoms in a polyatomic ion together? What kind would hold two polyatomic ions together? Type of bond? – Ionic, Polar Covalent, or Nonpolar Covalent? TiO2 CH4 NaI CS2 O2 KCl CsF HBr AlCl3 Types of Bonds 3. Metallic bond Metals bonding with other metals do not gain or lose e- or share e- unequally. These bonds are created from the delocalized e- that hold metallic atoms together. Chemical Formulas • A chemical formula is a combination of symbols that represents the composition of a compound. • Chemical symbols are used to indicate types of elements present. • Subscripts are used to indicate the number of atoms for each element present. What are the “parts” of a formula? chemical symbols C8H18 number of atoms of each element • 8 atoms of carbon • 18 atoms of hydrogen Charges of Monatomic Ions • Because atoms want to reach an octet of valence electrons, the oxidation numbers, (positive or negative charges) can be predicted for single atoms (monatomic). • Metals tend to have positive oxidation numbers. (lose e-) • Nonmetals tend to have negative oxidations numbers. (gain e-) 0 1+ 2+ 3+ varies +1 to +7 varies 3- 2- 1- Oxidation Numbers (charges) of Polyatomic Ions • Polyatomic ions are ions that are made up of two or more atoms. • Refer to your table of Polyatomic ions. • Polyatomic ions generally have the following endings: “ate” or “ite” Ex: NO2- nitrite PO43- phosphate SO42- sulfate Polyatomic Ions Oxidation Numbers • The sum of the oxidation numbers in a compound must equal zero. Ex: CaCl2 = Ca+2 + Cl- + Cl2 positive charges – 2 negative charges = 0 • The charge on a monatomic ion is its oxidation number. Ex: Ba+2 has an oxidation of +2 Cl- has an oxidation of -1 What happens when the predicted charge can vary?? The oxidation number of a transition element is shown using Roman numerals to indicate the charge. The Roman numeral indicating oxidation. Ex: iron (II) is Fe+2 iron (III) is Fe+3 Why is aluminum oxide Al2O3? Writing Ionic Formulas Ionic compounds are composed of metals and nonmetals. • Ionic compounds are made from the gaining or losing of electrons and the resulting electrostatic force that holds the ions together. • The sum of the oxidation numbers in a compound must equal zero. Writing Ionic Formulas • When writing formulas, the cation (metal ion) is always written before the anion (nonmetal ion). • When using polyatomic ions, refer to charge given on your table. NOTE: There is only one polyatomic cation (NH4+). The rest are all are polyatomic anions. Criss Cross Method of Writing Formulas Notice a trend between the oxidation/charges of ions and the subscripts of elements. Ex: Mg+2 and Cl- gives MgCl2 Ex: Al+3 and SO4-2 gives Al2(SO4)3 Criss Cross Method of Writing Formulas • This method crosses charges and subscripts to form neutral compounds. Al +3 and O-2 Al and O Al2O3 (neutral) Criss-Cross Method of Writing Formulas Ex: Lead (II) phosphate Pb+2 and PO4-3 Pb and PO4 Pb3(PO4)2 Nomenclature • Nomenclature is defined as a naming system. • Chemistry uses nomenclature to standardize names of chemicals. • Let’s take a look. Naming Binary Ionic Compounds Rules for naming binary (composed of two) ionic compounds: 1. Name of cation is given first. The name of the cation is the same as the element. 2. Name of anion is given last. The name of the anion is the same as the element, but with an “ide” suffix. Naming Ionic Compounds Ex: Al2O3 Aluminum and Oxygen Aluminum oxide cation anion Naming Ionic Compounds Ex: Ni2O3 Note that the cation has MORE THAN ONE possible oxidation state, so Roman numerals are needed to identify the ion. Nickel and Oxygen +3 -2 Nickel (III) oxide Naming Ionic Compounds EX: AgCl is __________________ Silver chloride Na2O is __________________ Sodium oxide CaBr2 is ___________________ Calcium bromide PbO2 is ___________________ Lead (IV) oxide Naming Ionic Compounds with a polyatomic ion Rules for naming compounds that contain a polyatomic ion. 1. Cation rule from binary applies. 2. Anion takes the name of the polyatomic ion as found on the table. Ex: Al2(SO4)3 aluminum sulfate Ex: Mg(OH)2 magnesium hydroxide Naming Ionic Compounds Ex: Li2CO3 is ____________________ Lithium carbonate Ba(OH)2 is ___________________ Barium hydroxide Zn(NO3)2 is __________________ Zinc nitrate KClO3 is ___________________ Potassium chlorate Naming continued… • Name the following compound: • Ba(Na)2 Banana Naming Binary Molecular Compounds • Unlike ionic compounds, molecular compounds are composed of individual covalently bonded units, or molecules. • Covalent compounds are formed between nonmetals. • Prefixes are used to indicate number of each type of element in the compound. • Write the prefixes as indicated on the next slide…. Molecular Prefixes 1 mono- 6 hexa- 2 di- 7 hepta- 3 tri- 8 octa- 4 tetra- 9 nona- 5 penta- 10 deca- (decade) Naming Binary Molecular Compounds Follow these rules: 1. The element written first is given a prefix if it contributes more than one atom to the molecule. 2. The second element is named by combining (a) a prefix indicating the number of atoms contributed by the element, (b) the root of the name of the second element, and (c) the ending -ide. 3. The o or a at the end of a prefix is usually dropped when the word following the prefix begins with another vowel. Ex: monoxide or pentoxide Naming Binary Molecular Compounds Ex: P4O10 1. P has more than one atom in this molecule. Tetraphosphorus 2. O is named by combining prefix, root name, and -ide ending. decoxide (a is dropped from prefix) *combine to form: Tetraphosphorus decoxide < TARGET="display"> Naming Binary Molecular Compounds Ex: SO3 is ____________________ sulfur trioxide PBr5 is ____________________ phosphorus pentabromide ICl3 is _____________________ iodine trichloride H2O is _____________________ dihydrogen monoxide Sb2O3 is _____________________ diantimony trioxide (*metalloid) • THE END Strange Names for Molecules BUCKMINSTER FULLERENE MORONIC ACID Paper Chromatography.mov Chromatography Lab Paper Chromatography Paper chromatography is a method chemists use to separate compounds from one another, but not change them. In this lab we will explore how this separation is made using different dye compounds. Molecules with similar polarities or molecular structures are attracted to each other. Water molecules have a polar structure. Because of this structure the oxygen end of the molecule has a small negative electrical charge and the hydrogen end has a small positive charge. Liquid water is held together by the attraction between the charges on different molecules. • A more complex, yet still similar molecule is cellulose, a molecule which is the basic component of paper. It is a very long molecule (a polymer) in which thousands of rings of six atoms each are linked together like beads. A portion of a cellulose molecule is shown below. • Paper Chromatography Paper chromatography is a method chemists use to separate compounds from one another, but not change them. The polar regions of these molecules are attracted to polar regions of the cellulose chains (which help to hold the fibers together in paper). Not surprisingly, water molecules, being polar, are also attracted to these regions and when paper is wet it loses strength because the water molecules get between the cellulose chains and weaken the attraction between them. • When water molecules move up paper that is dipped in water, the molecules which might be dissolved in the water will also be carried along up the paper. This is applied to the separation of dyes in a technique known as paper chromatography. •A spot of dye is placed on the paper above the level of the water. As the water moves up, the dye molecules will move with it if they are more strongly attracted to the water molecules than to the paper molecules. If the dye molecules are more strongly attracted to the paper than to the water, they will move more slowly than the water or even not at all. •What if the dye is a mixture? If two or more dyes have been mixed, then they may move at different rates as the water moves up the paper. If this happens, they will separate and we can identify them . This is depicted in the sketches below. Yellow #5 Structure • C16 H9 N4 Na3 O9 S2 • Skittles ingredients: sugar, corn syrup, hydrogenated palm kernel oil, apple juice from concentrate, citric acid, dextrin, natural and artificial flavors, gelatin, food starch, coloring (includes Yellow 6 lake, Red 40 lake, Yellow 5 lake, Blue 2 lake, Blue 1 lake, Yellow 5, Red 40, Yellow 6, Blue 1), ascorbic acid (Vitamin C). • M&M ingredients: Milk Chocolate (Sugar, Chocolate, Cocoa Butter, Skim Milk, Milkfat, Lactose, Soy Lecithin, Salt, Artificial Flavors), Sugar, Cornstarch, Corn Syrup, Dextrin, Coloring (Includes Blue 1 Lake, Red 40 Lake, Yellow 6, Yellow 5, Red 40, Blue 1, Blue 2 Lake, Yellow 6 Lake, Yellow 5 Lake, Blue 2), Gum Acacia. * The “lake” part means that the dye is attached as a coating