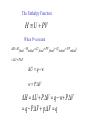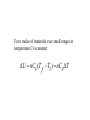* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Document
State of matter wikipedia , lookup
Thermal expansion wikipedia , lookup
Van der Waals equation wikipedia , lookup
Eigenstate thermalization hypothesis wikipedia , lookup
Determination of equilibrium constants wikipedia , lookup
Vapor–liquid equilibrium wikipedia , lookup
Gibbs paradox wikipedia , lookup
Temperature wikipedia , lookup
Degenerate matter wikipedia , lookup
Calorimetry wikipedia , lookup
Stability constants of complexes wikipedia , lookup
Equation of state wikipedia , lookup
Thermal conduction wikipedia , lookup
Transition state theory wikipedia , lookup
Heat transfer physics wikipedia , lookup
Equilibrium chemistry wikipedia , lookup
Chemical equilibrium wikipedia , lookup
Chemical thermodynamics wikipedia , lookup
Thermodynamics wikipedia , lookup
What is Thermodynamics 1. Understanding why things happens 2. Concerning heat, work, related temperature, pressure, volume and equilibrium 3. Equations relate macroscopic properties The laws of thermodynamics Number Basis Property Zeroth Law Thermal Equilibrium Temperature First Law internal energy, work and heat Internal Energy U Second Law Spontaneous process Entropy Third Law Absolute Zero of Temperature Entropy S0 as T0 Kelvin Study of heat engines Being studied by all students in physical science and engineering Concept of State V V (P,T ) From Avogadro’s hypothesis the volume per mole of all ideal gases at 0oC and 1atm pressure is 22.414 litres. PoVo 1 atm 22.414 litres To 273.16 deg ree.mole 0.082057 litre.atm / deg ree.mole PV RT R 0.082057 litres.atm / deg ree.mole 8.3144 joules / deg ree.mole For n mole gas PV=nRT For water vapour, the number of moles for Kg water is obtained by mass 1000 g n 55.56 mol 1 M 18 g.mol Thermodynamics Process Work and Energy Heat 1. Open system- material and energy exchange 2. Closed system- energy exchange only 3. Isolated system- no material and energy exchange What we learn from this module? 1.Internal energy U and entropy S 2.Combining U and S with P, T and V gives enthalpy H=U + PV and Gibbs energy G=H-TS 3.H is related to heat adsorption or release at constant pressure 4. G controls the position of equilibrium in closed systems at constant temperature and pressure. Why is Thermodynamics useful? 1.Qualitative explanation of materials behaviour 2.Quantitatively understanding of materials status. 3. Physical significance of thermodynamic functions. Applications of Thermodynamics 1.Extraction, refining 2.Corrosion 3.Phase transformation-phase diagram calculation 4.Materials processing 5.Design of new materials. The First Law of Thermodynamics Conservation of Energy Principle Same principle in mechanics, physics and chemistry U U U q w i f Work done in an Expansion (or contraction) against an External Pressure w Fx Pext F A F Pext .A w Pext Ax w PextV Pext .(V V ) 2 V ( final) w Pext dV V (initial) 1 Expansion against a constant external pressure v final w Pext dV P (v v ) P V ext final initial ext v initial W12 Q12 Reversible process and Maximum Work Reversible process for a closed system W system-environment =W environment-system Q environment-system =Q system-environment v final ws e P dV ext v initial v final we s P dV int v inital ws e we s wmax For an ideal gas P nRT int V v final w nRT dV V v initial For isothermal process, ie. T=constant wmax qrev nRT ln v( final) v(initial) Thermodynamics tutorial on the 24th of Oct (Tuesday) From 2pm D14 Questions*: 1. How to calculate W for a constant pressure process? 2. How to calculate W for an isothermal reversible process? 3. Is a constant pressure process an reversible process? Explain why? * All of these questions are concerned with ideal gas systems. Example: Gas compress during quasi-equilibrium processing, with PV=constant. The system is the gas P1=101325 N/m2, V1=0.01m3, V2=0.005m3 Find W W=-702J Enthalpy U=q-w · Q Heat is transferred due to the presence of a temperature difference. · Work here is considered as the work of expansion. · U results from the oscillation of atoms or ions in solid and movement of the particles in gas and liquid. · Q and w depend on how the change is carried out where the difference between them does not. · At constant volume, w=0 and U=q Thursday lecture • Sackville Street Building C9 • 11am-12:00 The Enthalpy Function H U PV When P=constant H H final H initial (U final PV final ) (U initial PV ) initial U PV U q w w P.V H U P.V q w P.V q P.V p.V q At constant pressure, the change in enthalpy is equal to the heat The change of enthalpy is independent of path. Q: Does q or W depend on path? For the change involving solids and liquids, HU, but for gases, HU Q:explain why? Example 1: Given p=constant=101.3 kPa V1=1m3, V2=2m3 Q12=200kJ Find a) U b) an expression for Q12 in terms of thermodynamics properties for a quasiequilibrium process. Example 2. Given T=T1=T2=constant for the process P1=200 kPa, T1=300K V1=2m3, V2=4m3 Find a) W and b) Q W=277KJ Heat Capacities (Cp and Cv) C q T a) Under constant volume conditions Cv- all heat supplied increases energy of sample b) Under constant pressure conditions Cp- Heat supplied increases energy of sample and provides energy for work performed. q Cv T Relation between Cv and U The 1st Law U q w When V=constant w0 Therefore U q U Cv .T Cv U T v U T v For n moles of materials over small ranges in temperature Cvconstant U nCv (T Ti ) nCv T f Relation between Cp and H At constant pressure Cp q T H C p .T Cp H T Cp H H T T p H C p T Over small range of T for n moles of materials H nC p (T Ti ) nC p T f Summary At constant volume q U At constant pressure q H Molar heat capacity at constant pressure H T P C p Molar heat capacity at constant volume U Cv T v Questions: 1. For a constant temperature process of an ideal gas, prove H=U. 2. For a gas system, explain why Cp is larger than Cv? 3. For a solid/liquid system, explain why Cp is close to Cv? 4. What are the equations for calculating change of enthalpy and internal energy due to temperature change?