* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Hot Topics in Liver Disease Wilson Disease a-1
Survey
Document related concepts
Transcript
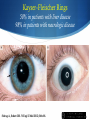
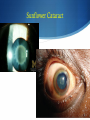
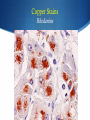
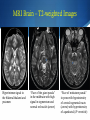
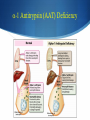
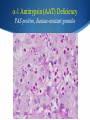
Hot Topics in Liver Disease Wilson Disease a-1-Antitrypsin Deficiency Norman L. Sussman, MD Baylor College of Medicine St. Luke’s Center for Liver Disease S Copper Absorption and Excretion • • • CMT1: Copper membrane transporter 1 ATOX1 Metallothionein Copper is a cofactor for a number of enzymes Ceruloplasmin (CPN), cytochrome c oxidase, dopamine b- hydroxylase, superoxide dismutase, tyrosinase ATP7B (P-type ATPase) Catalyzes self-phosphorylation of aspartate in the Cu2+ pump Excretes copper into the canaliculus Attaches copper to ceruloplasmin Samuel Alexander Kinnier Wilson 1878 – 1937 Born in Cedarville, NJ, moved to Edinburgh at one year of age after the death of his father Graduated with MB from University of Edinburgh in 1902 Trained in Paris with Pierre Marie and Joseph Babinski Returned to King’s College in London MD in 1912: "Progressive lenticular degeneration” and introduced the word “extrapyramidal” Question 1 Which of the following is true of Wilson disease (one answer): A. Autosomal dominant disease. 47% B. Is caused by a mutation in the ceruloplasmin gene. C. Is caused by a mutation in the ATP7B gene. 23% 17% 13% D. Is always associated with discoloration of the cornea (Kayser-Fleishcher rings). A. B. C. D. The Genetic Basis of Wilson Disease Hepatoleticular Degeneration Autosomal Recessive Copper storage disease – caused by defective copper export Accumulates in the liver, brain, cornea Not added to ceruloplasmin shortened half-life (low serum level) Not added to AP low level Mutations in ATP7B - multiple mutations The gene was identified in 1993 Prevalence ~30/million population Kayser–Fleischer Rings 50% in patients with liver disease 98% in patients with neurologic disease Schrag A, Schott JM. N Engl J Med 2012;366:e18. Question 2 Which of the following is not true (one answer): 40% A. Wilson disease may cause acute 37% hepatitis in children. B. Wilson disease may cause acute liver failure C. Adults with Wilson disease may 14% have no definite liver disease D. Neurological disease is the most 9% common presentation in newborns A. B. C. D. Wilson Disease Clinical Manifestations Hepatic, neurologic, psychiatric, hemolysis (ALF) – presentation may vary, even within a family Liver: 18-84% Neurologic: 18-73% Psychiatric: 10-100% Usually present at 5-35 years (up to 70s) Children typically present with liver disease (9-13 years) Older patients usually present with neurologic and/or psychiatric symptoms (15-21 years) Wilson Disease Clinical Manfestations Mild: abnormal liver tests, hepatomegaly, NAFLD Advanced: cirrhosis (with all complications) Acute liver failure (ALF) Neurologic Movement disorders (arms, gait, swallowing) Psychiatric Sunflower Cataract Wilson Disease – Diagnosis Classic presentation (about 50% of patients) 5-40 years old, cirrhosis, neurological findings, K-F rings, low ceruloplasmin Genetic diagnosis – better for confirmation Expensive Not universally available Sometimes inconclusive The rest – scoring system Wilson Disease – Diagnosis Very low ceruloplasmin (CPN) is strongly predictive Uric acid – usually low, but not validated Non-CPN bound copper is usually >25 mg/dL (250 mg/L) in untreated patients (normal = 15 mg/dL) Binding is ~3.15 mg of Cu2+ per mg CPN Free Cu2+ = serum Cu2+ (mg/dL) – 3x CPN (mg/dL) Google: ceruloplasmin-free copper calculator Caveat: may be elevated in chronic cholestasis, copper intoxication, and ALF of any cause Wilson Disease – Diagnosis 24-Hour Urine Copper Diagnostic at 100 mg/24 hours (1.6 mmol/24 hours) in symptomatic patients Many labs use 40 mg as the cutoff May be <100 mg at presentation in 16%-23% Penicillamine challenge (500 mg at 0 and 12 Hr) 1,600 mg (25 mol) copper/24 hours Validated in children, but not adults Wilson Disease – Diagnosis Liver Biopsy Early: mild steatosis (micro and macrovesicular) May show classic histological features of AIH Cirrhosis – macronodular and micronodular May be absent, even with neurological disease Copper stain is unreliable (metallothionein-bound) Absence of stainable copper does not exclude WD ALF – massive necrosis on a background of cirrhosis Copper Stains Rhodanine Electron Microscopy Wilson disease with steatosis Mitochondria vary in size and shape Increased density of the matrix Inclusions including lipid and fine granular material (copper) Pathognomonic increased intracristal space & dilatation of the tips of the cristae Elongated mitochondria and dense deposits. X15,000 Iancu & Manov, Technion, Haifa Wilson Disease – Diagnosis Hepatic Parenchymal Copper Concentration Copper copper content >250 mg/g dry weight 8% were <250, and all were >95 mg/g* Heterozygotes rarely exceed 250 mg/g Chronic cholestasis and copper intoxication Heterogeneous distribution – biopsy size Copper stain – unreliable (metallothionein-bound) Radiocopper labeling – rarely performed *Merle U, Schaefer M, Ferenci P, Stremmel W. Gut 2007;56:115-120 MRI Brain – T2-weighted Images Hyperintense signal in the bilateral thalami and putamen “Face of the giant panda” in the midbrain with high signal in tegmentum and normal red nuclei (arrow) “Face of miniature panda” in pons with hypointensity of central tegmental tracts (arrow) with hyperintensity of aqueductal (4th ventricle) Genetic Analysis Screening after the proband is diagnosed Over 300 mutations around ATP7B Not all gene changes cause Wilson Disease Single predominant mutation in some populations Sardinia, Iceland, Korea, Japan, Taiwan, Spain, Brazil and Canary Islands Eastern Europe –H1069Q mutation Wilson Disease – Therapy Uniformly Fatal Without Therapy British anti-Lewisite (BAL, dimercaprol) – introduced in1951 Developed to treat arsenic-based chemical warfare agent Still used for metal poisoning (arsenic, antimony, gold, etc.) Free sulfhydryl groups binds copper Wilson Disease – Therapy D-penicillamine – John Walsh, 1956 Chelates, induces metalothionein, prevents collagen crosslinking, immunosuppressant Initial worsening of neurologic symptoms in 10-50% Intolerance – 30% of patients Early – fever, eruptions, lymphadenopathy, neutropenia, thrombocytopenia Late – lupus-like (ANA+) – renal injury (hematuria, proteinuria), skin lesions Monitor with 24-hr urine and serum free copper 1,000 mg (16 mmol) (early) to 200-500 mg (3-8 mmol)/24h Non-ceruloplasmin copper Non-compliance vs inadequate chelation Wilson Disease – Therapy Trientine (triethylene tetramine dihydrochloride or 2,2,2-tetramine, trien Polyamine-like structure - copper forms a stable complex with 4 nitrogens in a planar ring Neurological worsening less common then with penicillamine Do not co-administer with iron: Chelates iron toxic complex Check effectiveness as for penicillamine Non-adherence: non-ceruloplasmin bound copper is over 150 mg/L Wilson Disease – Therapy Zinc Zinc induces enterocyte metallothionein which has a greater affinity for copper than for zinc binds copper in the enterocyte feces Used for maintenance or early cases – less toxic and more specific than chelating agents Dosed in mg elemental zinc – 50 mg TID for adults Wilson Disease – Therapy Ammonium Tetrathiomolybdate Strong de-coppering agent – two mechanisms: Interferes with intestinal uptake if taken with meals Binds copper from plasma if taken between meals TM remains an experimental therapy in the USA Does not cause neurological deterioration And Now for Something Completely Different Question 3 Which of the following is true of AAT A. The null mutation causes severe liver disease B. A mutation in one AAT gene may predispose a patient to alcoholic liver disease C. Liver disease and lung disease are both caused by low circulating levels of the enzyme D. Patients with the SS phenotype are particularly predisposed to liver disease 35% 26% 23% 16% A. B. C. D. a-1 Antitrypsin (AAT) Deficiency A Tale of Two Diseases Common in Caucasians – 1-3% of pts with COPD ~ 25 million people carry at least 1 deficient gene. The mutant protein is not secreted Liver: gets stuck in the hepatocyte injury Lung: Inadequate protection from leukocyte proteases e.g. trypsin, elastase, proteinase 3 May affect neonates (hepatitis) or adults (CLD, COPD) a-1 Antitrypsin Serpins – enzyme inhibitors Acute phase protein 52-kDa protein - 394 amino acids Produced in the liver – secreted into blood Complex folding – globular tertiary structure Neutrophil elastase binding site a-1 Antitrypsin (AAT) Deficiency a-1 Antitrypsin (AAT) Deficiency Pair of genes at the protease inhibitor (Pi) locus. The SERPINA1 (formerly Pi) gene on chr. 14 More than 100 allelic variants – classified based on serum levels of AAT protein. M alleles (Pi MM) are the most common Normal serum level Most patients with clinical disease are homozygous ZZ or SS or heterozygous MS, MZ, or SZ a-1 Antitrypsin (AAT) Deficiency PAS-positive, diastase-resistant granules a-1 Antitrypsin (AAT) Deficiency Normal is Pi MM – mutant alleles are S, Z, null Patients with liver disease typically have Pi ZZ Liver disease has no relationship to serum AAT activity May progress to cirrhosis and/or HCC No specific therapy Cirrhosis and HCC are indications for liver transplantation a-1 Antitrypsin (AAT) Deficiency Frequency Country Sweden Pi ZZ 0.06% Pi MZ -- Pi SZ 0.05% Year 1976 Netherlands France Spain 0.03% 0.01% 0.02% -2% -- 0.04% 0.15% 0.2% 1980 2003 2003 Italy Portugal Australia New Zealand 0.02% 0.02% 0.02% 0.02% 2.4% 2.5% 2.7% 4.5% 0.06% 0.3% 0.13% 0.17% 2003 2003 2003 2003 Canada 0.02% 2.7% 0.11% 2003 Kok et al. Netherlands Journal of Med, 2007 a-1 Antitrypsin (AAT) Deficiency Frequency Region Pop/No. Tested Pi MZ Pi MS Healthy donors/212 5 (2%) 7 (3.3%) Minneapolis, MN Blood donors/904 25 (2.8%) 37 (4.1%) Tucson, AZ General pop/2,944 88 (3.0%) 208 (7.1%) Long Beach, CA 7th-graders/1,380 34 (2.5%) 110 (8.0%) Los Angeles, CA Blood donors/2,010 59 (2.9%) 95 (4.7%) Rochester, NY Consecutive/500 18 (3.6%) 30 (6.0%) Rochester, NY Random pts/930 21 (2.3%) 60 (6.4%) St. Louis, MO Blood donors/1,933 42 (2.2%) 130 (6.7%) 10,813 292 (2.7%) 670 (6.2%) Rochester,MN Total Graziadei IW et al. Hepatology 1998 (4):1058-63. a-1 Antitrypsin (AAT) Deficiency Mayo: 641 OLT patients (Mar 1985 - Dec 1996) Phenotyping in 599 patients listed for liver transplant – examined for the Z allele 51 patients identified, 49 with MZ allele (8.2%) 2x frequency reported in the US population (2-4%) Pi MZ found in 27% with cryptogenic cirrhosis Graziadei IW et al. Hepatology 1998 (4):1058-63. a-1 Antitrypsin (AAT) Deficiency Heterozygotes Liver Disease Swedish Study (1972-1974) – 200,000 Neonates Screened 120 Pi ZZ (0.06%), 2 Pi Z-, 54 Pi SZ and 1 Pi S Only 14 Pi ZZ had prolonged jaundice – nine with severe disease All appeared healthy at six months of age Infants with a Pi SZ phenotype had no signs of liver disease. At 16 years, elevated liver enzymes were found in 17% of Pi ZZ adolescents and in 8% of Pi SZ Adults with liver disease in infancy were clinically healthy Kok et al. Netherlands Journal of Med, 2007 a-1 Antitrypsin (AAT) Deficiency Heterozygotes Liver Disease Northern Itlay – Phenotyping in umbilical cord blood Early childhood: Pi SZ: 5% had elevated enzymes Pi MZ: 7% had elevated liver enzymes At the age of 5 and 10 years, none had liver disease. Kok et al. Netherlands Journal of Med, 2007 a-1 Antitrypsin (AAT) Deficiency Liver Transplantation – Mayo Clinic 19872012: 5,246 patients 73 patients – 50 with ZZ and 23 with SZ phenotype Mean age 52.8 years, 76% men Pre-OLT AAT levels were lower in ZZ than SZ patients 28.3 vs 58.0 mg/dl, p=0.0001 Coexistent liver disease 8% in ZZ 43% in SZ 90% in MZ Carey et al. Liver Transplantation, 2013 - epub Summary Wilson disease – copper storage Caused by defect in ATP7B – low CPN Workup includes CPL, copper levels, biopsy Treatment with chelating agents and/or zinc AAT deficiency Pi ZZ may cause liver disease and HCC Pi MZ and Pi SZ may predispose to liver injury No treatment is available for liver disease besides liver transplantation