* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Nov10 Lecture 20 Evolution & vaccines
Oncolytic virus wikipedia , lookup
Virus quantification wikipedia , lookup
Endogenous retrovirus wikipedia , lookup
Introduction to viruses wikipedia , lookup
Plant virus wikipedia , lookup
Social history of viruses wikipedia , lookup
History of virology wikipedia , lookup
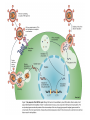
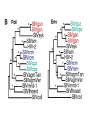
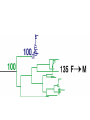
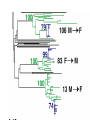
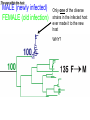
Lecture 21 Case study: influenza virus and Case study: HIV/AIDS Today: • Learning from the past to predict the future of influenza • The causes and consequences of HIV evolution • The “glycan shield” and within-host and between-host evolution of HIV Global impact of flu • Flu is a highly contagious respiratory illness which infects millions of people every year and kills hundreds of thousands • Caused by influenza viruses (A, B, C) • Estimated to infect 100 million people each year in the northern hemisphere alone • Huge impacts on morbidity and mortality, but also economic impacts Global impact of flu • Pandemics occurred in 1890, 1918, 1957, and 1968. The 1918 Spanish flu epidemic probably infected about 50% of the human population and represents the most intense culling of humans, ever. • It is very likely that pandemic influenza will return • Evolutionary tools can help fight currently circulating influenza, and possibly dampen the effects of future pandemic strains • Antigenic drift versus antigenic shift What is influenza? • There are 3 main types of influenza virus: A, B, and C • We’ll concentrate on influenza A, the most important from the human standpoint • Negative-stranded RNA viruses with segmented genome • 8 RNA segments encoding around 10 proteins What is influenza? • 2 glycosylated proteins on the surface, HA (hemagglutinin) and NA (neuraminidase) • HA and NA are involved in virus attachment and release from hosts cells • They are the primary targets of the immune system in humans (and swine) • Different strains of influenza are typically named for their HA and NA genes, eg. “H1N1” What is influenza? • The virus is capable of generating a lot of genetic variability • First, like other RNA viruses, the lack of proofreading and high error rate of the viral polymerase leads to high mutation rate. • This high mutation rate, in turn, leads to a high substitution rate. • (Substitutions are mutations that have become fixed through genetic drift or natural selection) • When these substitutions occur in antigenic epitopes, they can lead to escape mutants (antigenic drift) What is influenza? • The segmented nature of the influenza genome leads to another, more dramatic source of variability • Reassortment can occur when one host is co-infected with two different strains, and the progeny viruses get some gene segments from one “parent” and some from another • For example, if you were infected simultaneously with both H1N1 and H3N2, you might generate an H1N2 virus that could infect someone else and start a “new” epidemic Where does flu come from? • Reassortment gets particularly ugly when HA and/or NA genes that are new to the human population are introduced • There are 15 HA subtypes lurking in the gene pool of influenza that infects wild birds (H1-H15) • Birds are the reservoir of human influenza, the source from which new viruses periodically emerge via zoonosis • Importation of a variant to which few or no humans have prior immunity (antigenic shift) is the cause of the periodic pandemics Where does flu come from? • Since pigs can be infected with both avian and human influenza, and various reassortants have been recovered from pigs, it has been suggested that pigs might play the role of intermediary in the generation of reassortant pandemic strains • In 1979, for example, an avian influenza A began infecting swine in Northern Europe. This lineage has since clearly mixed with locally circulating human lineages, and has picked up human H and N2 HA and NA segment via reassortment Where does flu come from? • 1997, it became clear that avian influenza could also jump directly from birds into humans • The Hong Kong 1997 variant was an avian H5N1 virus that infected 18 people and killed 6 • Luckily, the virus was poorly transmissible in humans (if at all) • What would happen if someone got infected with avian H5N1 from their chicken, and also human H1N1 from their co-worker? Where does flu come from? • 1918 Spanish flu probably infected about 1 billion of the world’s 1.8 billion people, and led to the death of perhaps 50 million • Most deaths occurred in an 8-week window, October-November 1918 • Most deaths due to complications like pneumonia, dehydration • Unusual pattern of mortality, with healthy adults, 20-25, hardest hit Where does flu come from? Where does flu come from? Where does flu come from? Where does flu come from? • Painstaking work has been done to reconstruct the 1918 variant from archival specimens • No clear virulence factor was initially discovered • Recombination (as opposed to reassortment) was proposed as a solution, but that’s wrong Where does flu come from? • Recent structural studies of the HA protein of the 1918 virus revealed that, while maintaining many features of an avian virus, the structure of the HA allows it to bind to human cells without any trouble • So maybe the 1918 virus was the “perfect storm” in the sense that it represented a totally new gene, for which there was no standing immunity. But it could nevertheless replicate and transmit efficiently Where does flu come from? • It’s still not clear whether the virus jumped directly from birds or not • However, the children of 1918 may have been more accurate than anyone could have imagined • Further research should help answer remaining questions and inform surveillance and control measures Molecular clocks and natural selection Molecular clocks and natural selection Molecular clocks and natural selection Molecular clocks and natural selection Predicting the future of influenza • What is the expectation in the ratio of Dn/Ds if all changes are neutral? • What if changes to amino acids tend to be unfavorable? • What if changes to amino acids are favored? • Dn/Ds = 1: neutrality • Dn/Ds < 1: negative selection (a.k.a. purifying selection • Dn/Ds > 1: positive selection •Antigenic drfit due to mutations in the hemagglutinn gene necessitates frequent replacement of influenza A strains in the human vaccine •At least 18 of the 329 H3 HA1 codons have been under positive selection to change in the past •These showed a significant excess of nucleotide substitutions that result in amino acid replacements. •If the selective pressure on these was to evade immune responses of the host, then viruses with mutations at these codons should have been more fit •If true, could these patterns be used to predict which currently circulating strains will have highest fitness? •Tested “predictions” retrospectively •They defined fitness as follows: if one viral strain is more closely related to future lineages than another strain, regardless of virulence, it is more fit •Hence the goal of this work was not the same as predicting the epidemic strain for the next year, or predicting antigenic shift events • • • • Bush et al. used patterns of positive selection to predict “trunk” lineages in influenza A 18 codons in the HA gene of subtype H3 appear to be under positive selection Retrospective tests showed that lineages undergoing the greatest number of changes in those codons were the progenitors of future H3 lineages in 9 of 11 recent flu seasons Could help identify most “fit” extant strains that arise due to antigenic drift •The positive selection method predicted correctly 9 years out of 11 •There was a significant overlap between the positively selected sites and the codons in or near antibody combining sites and the sialic acid receptor binding site •How could these results be used to control influenza? Understanding HIV evolution crucial for… •Reconstructing its origin •Deciphering its interaction with the immune system •Developing effective control strategies like drug therapy and vaccines •HIV can infect a variety of cell types, but AIDS results from depletion of CD4+ T-helper lymphcyte cells •The env gene codes from the glycoproteins of the outer envelope of the virus •The gag (group-specific antigen) gene encodes the components of the inner capsid protein •The pol (polymerase) gene codes for the enzymes, including reverse transcriptase, that are used in viral replication •Which gene evolves the fastest? •Recombination plays a large role at all levels of HIV diversity •Including the origin of SIVcpz… •Evolution within and among hosts •Bottlenecks at transmission reduce diversity •But could the bottleneck have an adaptive explanation? •Phylogenies revealed that HIV continually replicates even when undetectable. How? •Heterosexual transmission accounts for most HIV infections worldwide, so understanding its ground rules is very important •Frequency of infection per coital act in less than 0.5%, so it’s pretty inefficient •Why? •Low amounts of virus? •Restricted access to target cells? •Selective transmission of a minority of variants? •Selective outgrowth of minority of variants? •Mother-to-infant transmission studies first showed that a restricted subset of viruses was observed soon after infection •Studies of sexual transmission have suggested that homogeneous, macrophage-trophic strains generally establish infection •Derdeyn et al systematically examined the properties of viruses transmitted in a series of FTM and MTF transmission pairs •Large cohort of HIV-discordant cohabiting couples in Zambia (one has HIV, one doesn’t, at start) •Eight couples out of >1000 showed HIV transmission •Blood samples collected simultaneously from both couples with a few months of transmission…. •Recipient viruses were monophyletic, nested within donor variation •They tended to encode compact, glycan-restricted envelope glycoproteins •This suggest that variants within a donor host that have not evolved changes in their env genes that code for a glycan shield are either transmitted more effectively, or outcompete other variants when they get transmit to a new host? •These variants were uniquely sensitive to neutralization by antibody from the transmitting partner •The exposure of neutralizing epitopes, which are lost in chronis infection because of ongoing immune escape mutation/selection, appears to be favored in the newly infected host •Implications for vaccine design? The war within the host The officers normally give orders to release mustard gas and send troops into battle QuickTime™ and a TIFF (Uncompressed) decompressor are needed to see this picture. antibodies Elimination of the officers leaves entire armies wandering aimlessly, and any invasion becomes successful QuickTime™ and a TIFF (Uncompressed) decompressor are needed to see this picture. Killer T-cells The war within the host •Heterosexual transmission accounts for most HIV infections worldwide, so understanding its ground rules is very important •Frequency of infection per coital act in less than 0.5%, so it’s pretty inefficient •Why? •Low amounts of virus? •Restricted access to target cells? •Selective transmission of a minority of variants? •Selective outgrowth of minority of variants? The war within the host •Derdeyn et al systematically examined the properties of viruses transmitted in a series of FTM and MTF transmission pairs •Large cohort of HIV-discordant cohabiting couples in Zambia (one has HIV, one doesn’t, at start) •Eight couples out of >1000 showed HIV transmission •Blood samples collected simultaneously from both couples with a few months of transmission…. The war within the host MALE (newly infected) FEMALE (old infection) Only one of the diverse strains in the infected host ever made it to the new host WHY? The war within the host •Variants without a “sugar shield” are either transmitted more effectively, or outcompete other variants when they transmit to a new host •Transmitted variants appear to be easier to control with antibodies •Implications for vaccine design? The war within the host QuickTime™ and a TIFF (Uncompressed) decompressor are needed to see this picture. The war within the host QuickTime™ and a TIFF (Uncompressed) decompressor are needed to see this picture.