* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Document
Genome (book) wikipedia , lookup
Minimal genome wikipedia , lookup
Gene expression profiling wikipedia , lookup
Protein moonlighting wikipedia , lookup
Primary transcript wikipedia , lookup
Gene therapy of the human retina wikipedia , lookup
Artificial gene synthesis wikipedia , lookup
Point mutation wikipedia , lookup
Oncogenomics wikipedia , lookup
Epigenetics of human development wikipedia , lookup
Therapeutic gene modulation wikipedia , lookup
Vectors in gene therapy wikipedia , lookup
Mir-92 microRNA precursor family wikipedia , lookup
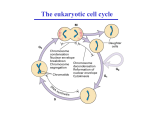
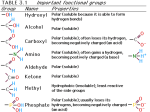
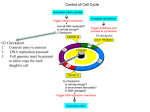
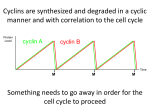
Lectures 7 -10 Cell Cycle Regulation and Cancer Introduction to the Cell Cycle The Eukaryotic Cell Cycle and Chromosome Dynamics Identification and Experimental Basis of Cell Cycle Regulatiory Factors Major players: • Cyclins , cdk’s (cyclin – dependant kinases) • MPF (Maturation Promoting Factor) = cyclin Bcdc2 (yeast) or cdk1 kinase (“mitotic cdk”) • CAK (Cdk Activating Kinases) regulators of cdks • CKI – cdk inhibitory subunits • APC (Anaphase Promoting Complex) and cdc34/SCF ubiquitination pathways which activate proteolysis via proteosomes. Introduction to the Regulation of Cell Cycle Also termed SCF pathway Identification and Experimental Basis of Cell Cycle Regulatory Factors contd… Mutant studies in Saccharomyces cerevisiae (budding yeast) and Schizosaccharomyces pombe (fission yeast) Complementation studies of the cdc (cell division cycle) temperature sensitive mutants using a plasmid library of wild type yeast DNA have enabled identification of numerous proteins involved in cell cycle regulation. Identification and Experimental Basis of Cell Cycle Regulatory Factors contd… Biochemical studies with oocytes: • Oocytes from Xenopus laevis, the South African clawed toad, are a store house for factors required for cell cycle progression & cell division from the oocyte stage to the fertilized egg through gastrulation during embryogenesis. • In vitro studies using this model system have led to the identification of various components and events in the cell cycle. This began with the identification of “MPF” (maturation promoting factor) which controls entrance into meiosis and mitosis. It was later shown to be the equivalent of the cyclin b-cdc2/cdk1 complex Functions of MPF (cyclin b-cdc2/cdk1) • Reversible breakdown of the NE & role of nuclear lamin proteins via DNA transfection experiments. • Genetic studies on S.cerevisiae identified a family of proteins that are required for normal chromosome segregation and are termed SMC ( Structural Maintenance Complex) proteins. They are found in organisms up to humans and are typically called “condensins”. • Phosphorylation of condensins by MPF or another protein kinase regulated by MPF is necessary for maintaining the condensed state of the chromosomes via DNA binding. • Phosphorylation of APC results in initiation of anaphase. Identification of MPF (Maturation Promoting Factor) and Cyclins in Xenopus Oocytes • Oocyte maturation to the meiotic II metaphase stage is studied by microinjection of the female steroid hormone, progesterone, into G2 arrested oocytes. • This can be mimicked by the microinjection of cytoplasm from the metaphase II stage. • The “factor” that mediates this oocyte maturation was purified and termed MPF. • MPF oscillates in coordination with meiosis in the oocyte and mitosis during early cleavage stages following fertilization. • This oscillation was due to the cyclic degradation and biosynthesis of cyclin B. • It was subsequently demonstrated that MPF is mitotic cyclin b-cdc2 or -cdk1 complex. G2-arrested oocyte Meiosis I Meiosis II Experimental Demonstration that the Synthesis & Degradation of Cyclin B are Required for the Cycling of MPF and Mitotic Events in Xenopus Egg Extracts Sperm Chromatin (mutation in destruction box sequence) Sperm Chromatin Polyubiquitination of Mitotic Cyclins • Regulated degradation of mitotic cyclins occurs in late anaphase via the APC-ubiquitin pathway which makes use of a “destruction box” sequence common to the N-T of mitotic cyclins. • This ubiquitin pathway involves E1 (ubiquitin activating enzyme) , E2 (ubiquitin conjugating enzyme) & E3 (ubiquitin ligase or APC). • Mutant genes that code for non-degradable cyclins have been produced by site directed mutagenic deletion of the destruction box. Regulation of Mitotic Cyclin Levels in Cycling Cells • Regulated degradation of mitotic cyclins is a result of a corresponding activation of APC activity via phosphorylation by mitotic cdk and Cdh-1 binding. • This is an example of two protein complexes which regulate each other in concert with cell cycle regulation. Cdh-1 cyclin B degrades Mechanism for the Role of APC in Initiating Anaphase • Anaphase is induced by degradation of the “anaphase inhibitor” (“securin”) via the APC (anaphase promoting complex) pathway activated by MPF (P) (cyclin B-cdk1) and Cdc20 addition to APC. • This protein is involved in the stabilization of a multicomplex of proteins called cohesins. Cohesins act as “linchpins” to tie together sister chromatids at the centromeric attachments and other sites along the chromatid pairs and maintain this association despite the pulling of the kinetochore attached spindle fibers. • Some of the cohesins are members of the SMC protein family and thus related to the condensin proteins which maintain chromosomes in a condensed state. • Degradation of anaphase inhibitor (securin) results in inactivation of cohesin function allowing the poleward force exerted on kinetochore to move sister chromatids towards opposite poles. • Destruction of the mitotic cyclin B via the APC pathway occurs in late anaphase when APC complexes with cdh-1. Model for Induction of Anaphase by Regulation of Cohesin Complexes Regulation of Sister Chromatid Pairing by the Cohesin Complex • Sister chromatid pairing is mediated by the cohesin complex in which Smc1/Smc3 together with Scc1/Scc3 form a bridge between the two chromatids. • Scc1 is the critical protein that serves as a “linker” between cohesin complexes on the two sister chromatids • APC/Cdc20 mediates release of the ‘anaphase inhibitor’ (“securin”) by activating securin degradation. This results in activation of separase and cleavage of Scc1 linkages between cohesin complexes Cdc20 Summary of the Dual Effect of APC on Mitosis P + Cdh-1 [Securin] + Cdc20 Active Separase breaks Cohesin links Cell Cycle Regulatory Components • S. pombe genetic studies especially complementation and recombination studies of the cdc temperature sensitive mutants led to the identification of cdc2 and cdc 13 as cdc2 kinase and cyclin b. • Both of them are linked in a complex as MPF or cyclin b-cdc2 kinase complex. • It also identified other regulatory components such as wee 1, cdc 25 and CAK (cdc2 activating enzyme). • Multiple pathways are involved in regulating the mitotic-cdk complexes which are cdc2 in yeast and cdk1 in mammals. • All these cell cycle regulatory proteins are typically well conserved from yeast to humans. Regulation Wheel of CDK’s (Morgan, Nature 374 (1995) 131-134) Tyr-15 P-Thr-161 The Regulatory Aspects of DNA Replication Genetic studies with S.cerevisiae have dissected the regulatory aspects of DNA replication: (a) Control of S-phase by regulated proteolysis of sic 1: (i) Sic 1 is a CKI specific for the S-phase cdk-cyclin complex. (ii) This proteolysis is initiated by the G1 phase cdk-cyclin which phosphorylates sic1 making it a target for cdc34 ubiquitin conjugating enzyme and a trimeric ubiquitin ligase called SCF. The Regulatory Aspects of DNA Replication contd… (b) Regulation of pre replication complexes: cdk’s simultaneously activate initiation of replication and prevent re-initiation at origin sites by phosphorylation & disassociation of McM’s from the ORCs (origin recognition complex) which are permanently associated with replication origins. For the McM’s to reassemble at the origins they need to be dephosphorylated which does not occur until mitosis. P P Cell Cycle Control in Mammalian Cells Multiple cdks and cyclins regulate passage of mammalian cells through the cell cycle. Cyclin D is Required for Passage Through the Restriction Point in the Mammalian Cell: An In Vivo Depletion Experiment Cells microinjected anti-cyclin D 8 h after growth factors Control DAPI An in vivo Depletion exp BrdU anti-Cyclin D microinjection Early and Delayed Genes and Regulation at the “Restriction Point” • Activation of Go cells into the cell cycle of growth and proliferation requires growth factor stimulation and involves transcriptional induction of early response and delayed response genes (DRG). • Induction of early response genes is not blocked by inhibitors of protein synthesis (e.g. cycloheximide) because the transcriptional factors that control these genes are already present in the Go cells and are activated by post transcriptional modifications e.g., phosphorylation via protein kinases activated by signal transduction systems activated by growth factors. • Many of the early response genes code for general transcription factors (TF) e.g. (c-Fos and c-Jun) that stimulate transcription of the delayed response genes (DRG). Some of the DRG encode additional components of cell cycle regulation, e.g., the cyclins and G1cdks. EF2 is a TF that regulates transcription of cyclins A & E and cdk2 genes. Mechanism for Restriction Point Regulation • If growth factors are withdrawn before the passage through the “Restriction point” (RP), transcription of the DRGs (e.g., G1 cyclins and cdks) rapidly decreases. • Since these proteins and the mRNAs encoding them are unstable , their concentrations in the cell fall rapidly. Thus the cells do not pass the RP and do not enter S-phase and thus do not proliferate. Role of E2F and Rb Protein in Regulation of RP Passage • The E2F transcription factor regulates the cyclin A & D and cdk2 genes. • Rb (retinoblastoma) protein inhibits E2F transcription factor. • Rb is the protein product of the tumor supressor gene RB which if mutated enhances the probability of retinoblastoma and other cancers. • Normally this protein is not active until mid/late G1 phase when the buildup of cdk2-cyclin E and cdk4/6-cyclin D leads to phosphorylation of Rb and release from supression of the E2F transcription factor. • After mitosis Rb is dephosphorylated due to the low concentrations of the G1 cdks and cyclins. Rb will undergo phosphorylation as the cyclin levels rise in G1. If not, the cell cycle goes into Go (i.e., it does not pass RP). Mid-G1 Late-G1 A + Cyclin Cdk2 release from RP Checkpoints in Cell Cycle Regulation To ensure orderly passage of the cells through cell cycle there are four defined “checkpoints” where certain events need to occur before the cell will proceed in the cell cycle; otherwise the cell will be “arrested” at that checkpoint: G1 arrest, S arrest, G2 arrest and M arrest. There are specific checkpoint protein kinases (chks) that are directly involved in arresting the cells. Role of p53 in G1 Checkpoint Arrest • Most commonly mutated tumor suppressor gene associated with human cancers is p53 which encodes the transcription factor (TF) p53 protein. Cell sorting Wild-type cells • This protein is rapidly degraded in normal cells so its concentration is very low. Cell sorting cells p53- mutant • In response to DNA damage in G1, the protein level of p53 rises dramatically due to the activation of a checkpoint protein kinase (chk2) that phosphorylate p53 and make it less susceptible to degradation by the ubiquitination/proteasome pathway • This enables p53 to activate transcription of the cdk inhibitor (CKI) p21 which binds to all cdks and inhibits their action and thus “arrests” the cells until the DNA damage can be repaired. Then the p53 fall back to normal levels and the cell is “rescued “ from checkpoint arrest. P P P (which activates a checkpoint kinase (phosphorylate p53) • Under severe DNA damage p53 activates genes that lead to apoptosis (cell death). Cancer and Growth Related Factors: ProtoOncogenes and Tumor Supressor Genes • Cancer is characterized by the ability to grow in absence of growth promoting factors and resistance to signals that stop growth or program cells for apoptosis. • Cancer is a disease of: unregulated growth, proliferation and cell to organ function. • Genes coding for these regulatory factors can be divided according to their cancer related properties into either: (a) Oncogenes or (b) Tumor Suppressor Genes Oncogenes • Oncogenes produce proteins that have the capacity to stimulate growth and proliferation. • They are dominant or “gain of function” mutations. • First discovered through the ability of Rous sarcoma virus (RSV) to cause cancer in chickens. • Mutant studies of RSV: the src gene causes cancer! • Transfection of cells with src or other oncogenes. e.g., ras or jun leads to neoplastic transformation. • There is a normal cell equivalent of this so-called “oncogene” and it codes for a protein that has been associated with tyrosine kinase activity, that stimulated growth and proliferation via protein phosphorylation in signal transduction pathways. Oncogenes contd… • The activity of the normal protein is regulated by another tyrosine kinase that phosphorylates src at T-527 (p-527 src is inactive). • v-src has a deletion in 18 aa at the C-T that includes T-527. • Therefore , v-src or appropriate cellular mutants (csrc) have constitutive tyrosine kinase activity, therefore unregulated cell growth and proliferation. • All oncogenes have been found in normal cell equivalent genes/proteins and are termed “protooncogenes”. Oncogene proteins • Growth factors – rare but an example is sis which codes for a mutant PDGF (platelet derived growth factor) and aberrantly auto stimulates proliferation of cells containing PDGF receptors. • Growth factor receptors- erb b is a mutant form of epidermal growth factor. This receptor functions as a tyrosine protein kinase (CT of protein) located on the cytoplamic side of the membrane with the ligand binding region (NT of protein) facing the cell exterior. In erb b the receptor protein is missing the N-terminal ligand binding domain on the cell surface exterior and the C-terminal tyrosine protein kinase is in a permanently turned on state. •Intracellular transducers e.g., ras (a mutant form of an inner cell surface GTPase) , whose function activates a number of other regulatory factors including another oncogene termed raf (a protein kinase) that work in concert to regulate cell proliferation via the MAP Kinase Pathway which activates transcription factors •Nuclear transcription factors like myc. The myc protein is a key factor involved in activation of gene transcription in cells going from a non – proliferating state. If myc gene expression is blocked using anti-sense oligonucleotides, cell cycle progression is stopped. Over expression of c-myc is characteristic of many cancers. Oncogenes Contribute To Cancer By Over Expression Of Normal Regulatory Components (1) Point mutations that lead to constitutively acting protein products. (2) Amplification of the gene or change in chromosomal location of the gene that puts the gene under the regulation of a different promoter or activator. Tumor Supressor Genes • Tumor supressor genes produce proteins that suppresses growth and proliferation. • These are “loss of function” or recessive mutations. • Being heterozygous enhances the probability of cancer but this will require a mutation in the corresponding other allele. e.g., it need to be homozygous for the gene. • Tumor supressor genes include: (a) Proteins that regulate or inhibit progression through a cell cycle stage like CKI’s: p16, p21; (b) Receptors for secreted hormones/growth factors that function to inhibit proliferation: e.g., TGF-beta; (c) Restriction point (Rb) & check point (p53) control proteins. Human Colorectal Cancer: Multi-Tumor Suppressors and Oncogenes • APC –tumor supressor gene inhibits ability of wnt protein to activate expression of the myc oncogene. • Absence of functional APC thus leads to an uncontrolled expression of myc oncogene protein that activates genes required for the G1-S-phase progression. • This leads to polyp (small tumors) formation along the colon wall. • Cancer progression can then proceed towards malignancy if subsequent mutations occur: ras oncogene, DCC-tumor suppressor gene, p53 tumor suppressor gene. The RB Gene and Heredity Retinoblastoma Loss of Heterozygosity (LOH) of Tumor Suppressor Genes Why do patients who inherit one mutant allele of RB have a high probability of developing retinal tumors in childhood? This is characteristic of cancers related to the loss of tumor suppressor genes and a likely explanation is the predisposition of such genes to a “lose of heterozygosity” or (LOH). Conclusion Cancer is characterized by many abnormalities in chromosome numbers (aneuploidy) and organization (translocations, inversions deletions, and amplifications). This is a direct result of the lack of checkpoint control during the cell cycle. CANCER CELLS ARE CHARACTERIZED BY GLOBAL ABERRATIONS IN THE NUMBER SIZE AND BANDING PATTERNS OF CHROMOSOMES GIVING RISE TO THE IDEA OF A “CHAOTIC GENOME” IN CANCER CELLS. Chromosome painting of an osteosarcoma cell line (MG-63) Chromosome painting of normal diploid human cells PLEASE DO NOT SMOKE