* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download document 8296386
Fatty acid synthesis wikipedia , lookup
Ribosomally synthesized and post-translationally modified peptides wikipedia , lookup
Citric acid cycle wikipedia , lookup
Peptide synthesis wikipedia , lookup
Genetic code wikipedia , lookup
Mitogen-activated protein kinase wikipedia , lookup
Biochemistry wikipedia , lookup
Paracrine signalling wikipedia , lookup
Biochemical cascade wikipedia , lookup
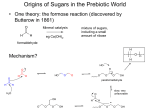
5.451 F2005 Saccharide Biosynthesis Deoxy sugar biosynthesis review Amination requires the presence of a keto group; otherwise timing not specified Me O O Me O Me PMP HO ONDP H2N tautomerization O O 4,6 dideoxy HO ONDP Me HO O PMP HO ONDP O ONDP Me HO H2N O � Desosimine --> erythromycin ONDP 5.451 F2005 Shikimate Pathway Normally usd in synthesis of aromatic amino acid Branch points from a primary metabolic pathway to make a variety of natural products 1. phenyl-glycine amino acids --> vancomycin --> comparison (incorporated peptide products) PKS 2. amino shikimate --> rifamycin --> PK product 3. cyclohexyl CoA --> avermectins --> incorporated into a PK product 4. coumaryl CoA derivatives for flavonoid biosynthesis starting materials 5.451 F2005 Shikimate Pathway transfer plant genes to e. coli + express S.A. in e.coli another technique 28g/L culture presence of a solid ion exchange resin 14% yield based glucose starting material Figure removed due to copyright reasons. Please see Scheme 1a in JACS 123 (2001): 10173-10172. based coumpounds accumulate Knock out transporter = leave shik. acid in media outside cell --> 52g/L 18% yield J 5.451 F2005 Shikimate Pathway Figure removed due to copyright reasons. Please see: Hubbard, Brian K., and Christopher T. Walsh. Scheme 2 in “Vancomycin Assembly: Nature's Way.” Angew Chem Intl Ed 42 (2004): 730-765. 5.451 F2005 Shikimate Pathway Figure removed due to copyright reasons. Please see: Hubbard, Brian K., and Christopher T. Walsh. Figure 6 in “Vancomycin Assembly: Nature's Way.” Angew Chem Intl Ed 42 (2004): 730-765. 5.451 F2005 Shikimate Pathway Angew Chem Intl Ed 42 (2003): 730-765. 5.451 F2005 Shikimate Pathway Angew Chem Intl Ed 42 (2003): 730-765. 5.451 F2005 Shikimate Pathway Angew Chem Intl Ed 42 (2003): 730-765. 5.451 F2005 Shikimate Pathway Ansa macrolides: incorporation of amino shikimate OH O H N O O O O O OH OH O H N OH O MeO N O O Rifamycin W OH MeO NH O OH HO Mitomycin O O Napthomycin MeO O NH2 MeO OH HO O O O O O O OH N H OMe O Geldanamycin NH OH O O H N S-CoA NH2 O O HO O O OH OH OH OH NH OH OH O OH HO Rifamycin W OMe O O O O O OH OH O Proansamycin X Ansatrienin A 5.451 F2005 Shikimate Pathway Amino Derivatives HO2C O3P O O3PO H HO O OH DAHP synthase CO2 HO CO2H CO2H CO2H CO2H O3PO O HO OH O OH O OH OH OH dehydroquinate synthase CO2 OH CO2H CO2H O3PO OH OH shikimate kinase shikimate dehydrogenase dehydroquinate dehydratase HO OH HO OH CO2H CO2H O3PO O NH2 HO OH O NH2 OH O NH2 OH enzymes of amino shik. require amine moiety for recognition HO NH2 OH HO NH2 amino shik. 5.451 F2005 Shikimate Pathway Amino Derivatives Copyright 2003 National Academy of Sciences, U.S.A. from PNAS(2003) 100, 9774-9778 5.451 F2005 Shikimate Pathway Amino Derivatives Copyright 2003 National Academy of Sciences, U.S.A. from PNAS(2003) 100, 9774-9778 5.451 F2005 Shikimate Pathway Cyclohexyl-CoA OMe HO O O OH O MeO O O NH O HO N O O MeO OH H O OMe O O NH Rapamycin (Ascomycin, FK506) O O Ansatrienin A 5.451 F2005 Shikimate Pathway Cyclohexyl-CoA Figure removed due to copyright reasons. Please see Figure 2 in J Indus Microbiol Biotech 20 (1998): 299-303. 5.451 F2005 Shikimate Pathway Adding on a cyclohexyl starter unit antiparasitic agents O H O O OH Avermectin S avermitilis O H OH O H O O O OH CoA-S O 2-methyl-butyryl CoA Doramectin S. collinus O O H OH CoA-S O 2-cyclohexyl CoA Figures removed due to copyright reasons. Please see Figure 3 in Nature Biotech 18 (2000): 980-983. f vector or a plasmid containg all cyclo hexyl biosyn genes 5.451 F2005 Shikimate Pathway Coumarin Figure removed due to copyright reasons. Please see Figure 1 in J Indus Microbiol Biotech 30 (2003): 456-461. 5.451 F2005 Shikimate Pathway Coumarin O O OH O S OH O S NH2 HO O S NH2 HO NH2 HO NH2 HO L-Tyrosine NovI A PCP NovH NovJ/K A PCP A PCP NovO OH H2N O O O OH OH O O OH OH O O Novobiocin HO O OH HO NH2 OH S HO NH2 NovC ? O 4-hydroxy-3-amino coumarin A PCP amino coumarin coumarin --> PKS | shik. O S NH2 H N O O A PCP 5.451 F2005 HO2C Shikimate Pathway O3biosynthesis P O Deoxy sugar O3PO HO CO2H CO2H CO2H CO2H O3PO O H HO CO2 OH HO O OH OH O OH O OH OH HO OH OH O3PO OH OH OH CO2H CO2 CoA-S O3PO O HO NH2 O O3PO O CO2 OH polyketide starter units OH CO2H O CO2H HO NH2 OH OH non-ribosomal peptide biosynthesis CO2 O2C H2N polyketide starter units O COOH OH flavonoids CO2 HO CO2H Tyr, Phe