* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Environmental Toxicology
Biochemistry wikipedia , lookup
Model lipid bilayer wikipedia , lookup
Cre-Lox recombination wikipedia , lookup
Cell culture wikipedia , lookup
Vectors in gene therapy wikipedia , lookup
Cell-penetrating peptide wikipedia , lookup
Basal metabolic rate wikipedia , lookup
Signal transduction wikipedia , lookup
Polyclonal B cell response wikipedia , lookup
Pharmacometabolomics wikipedia , lookup
Endomembrane system wikipedia , lookup
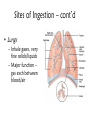
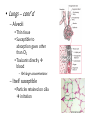
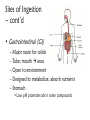
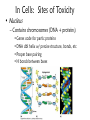
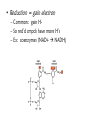
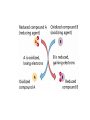
Environmental Toxicology Toxicants in Living Organisms Ingestion Excretion • Phys/chem properties impt – Forms • Gases, vapors (evap’d solvents), dusts • Liquids (in H2O) • Solids (dissolved) Ingestion Excretion • Phys/chem properties – cont’d – pH, pKa, solubility • Absorption effected? (ex: pH) – Effects toxicity – Ex: aspirin acidic, but neutral in stomach • Must be soluble in body/cell fluids for abs’n • Lipid solubility also impt – Cell membr mostly lipid Secobarbital Thiopental Introduction of Toxicants • Exposure – – – – Concentration, dose Duration, frequency Site, route Figure 5.2 • Variations – Species/strain differences – Genetic/health status – Environmental factors (light, temp, etc.) Sites of Ingestion • Skin – Mostly liquids, solutes in sol’n, suspensions – Greatest area: epidermal cells – blood, lymph body • Blood flow impt – Penetration depends on • Phys/chem properties of toxicant • Skin penetrability – In gen’l nonpolar agents enter Sites of Ingestion – cont’d • Lungs – Inhale gases, very fine solids/liquids – Major function – gas exch between blood/air • Lungs – cont’d – Alveoli • Thin tissue • Susceptible to absorption gases other than O2 • Toxicants directly blood – Rel large concentrations – Itself susceptible • Particles retained on cilia irritation Sites of Ingestion -- cont’d • Gastrointestinal (GI) – – – – – Major route for solids Tube: mouth anus Open to environment Designed to metabolize, absorb nutrients Stomach • Low pH promotes abs’n some compounds • GI – cont’d – Small intestine • Absorption – Enterohepatic circulation • Intestine blood liver bile gi blood • Liver – “Screening organ” Toxicant Storage • Fat – Lipophilic compounds • Many pesticides • Bone – Compounds that bind CaPO4 • Includes small ions In Cells: Sites of Toxicity • Nucleus – Contains chromosomes (DNA + proteins) • Genes code for partic proteins • DNA dbl helix w/ precise structure, bonds, etc • Proper base pairing • H bonds between bases • Nucleus – cont’d – Transcription • Many steps, proteins nec • DNA mRNA – Translation • Many steps, proteins nec • mRNA protein • Nucleus – cont’d – Toxicants may • Physically disrupt DNA helix • Disrupt repl’n process – Decr’d # new cells • Chem’ly alter bases – Improper base pairing – Mutations • ~ 500 diseases w/ 1 aa change – Often due to defect in genetic code Major Sites of Toxicants in Cells – cont’d • Enzymes – Proteins that catalyze cellular rxns – Proteins have partic structures • Based on aa’s that make them up • Can be disrupted by cell phys/chem changes • Enzymes – cont’d – Active site • Region holds substrate(s) by multiple weak chem. interactions • Atoms of aa side chains participate in rxn w/ substrate(s) • Rxn catalyzed by lowering energy nec for rxn to take place – Common mech of toxicants is destruction of enz’s, or disruption of their catalytic ability http://www.blobs.org/science/enzyme/imgs/active2.gif • Enzymes – cont’d – Toxicants may: • Bind covalently at enz active site or other site on enzyme • Compete for enz active site • Unravel enz folding • Enzymes (cont’d) – Toxicants may (cont’d): • Inactivate impt cofactor (inorganic ion nec for enz activity) – Form complex w/ cofactor » Book ex: enolase catalyzes 2phosphoglycerate phosphoenolpyruvate; req’s Mg+2 » Presence of F Mg-F-PO4 complex inact’n enz – Compete with cofactor » Book ex: Cd replaces Zn Major Sites of Toxicants in Cells – cont’d • Metabolic Processes – Mitochondria impt • Respiration – aerobic (O2) • Also, anaerobic – Anabolism/catabolism • Metabolic Processes – cont’d – Redox reactions • Shift electrons (1 mol loses e- as [H-] or [H+ + e-]; another gains) • Impt to ATP synth (cell energy) – Toxicants may • Alter enz’s impt to metab improper metabolite • Use metabolic enz’s for toxicant metab improper metabolite Major Sites of Toxicants in Cells – cont’d • Cell Membrane – Encloses cell – Mostly lipid – Receptor proteins • Lipophilic substances enter • Specific • Cell biochem rxns depend on these • Cell Membrane – cont’d – Toxicants may • Damage lipid bilayer • Damage receptors or shift their structures • Oxidize lipids • Smooth Endoplasmic Reticulum – Contains enzymes involved in metabolism of toxicants Toxicant Metabolism • Chem nature of toxicants – Extremes of acidity/basicity/ability to add or remove H2O • Corrosive, caustic compounds • Irritants • Very reactive toward mol’s in tissues • Chem nature of toxicants (cont’d) OH – Highly reactive substances • Bonds, functional groups easily react w/ biomolecules damage • Ex: allyl alcohol vs propanol • Ex: peroxides – Heavy metals • Many react w/ proteins (so enzymes) – May bind –SH grp (cysteine) • Chem nature of toxicants (cont’d) – Compounds that bind impt proteins • Reversibly or irreversibly • Ex: CO irreversibly binds Hb – Lipid-soluble compounds • Traverse lipid bilayer • Enter cells easily Metabolism – cont’d • Ingested toxicant may be – – – – Abs’d as parent Metab’d first, then abs’d Stored Excreted • In general, acted on by metabolic enz’s – Mistaken for food – “Biotransformation” • BUT nonenzymatic biotransformations also • Figure 10.2 – good summary – Dependent on phys/chem properties of xenobiotic • Highly polarized/ionized – Don’t enter cells – Rapidly excr’d – Little harm • Volatile – Expelled quickly from lungs – Little harm • Nonpolar (lipophilic) – Less soluble in aqueous body fluids – Attracted to body lipids – Can accumulate in tissues, fat Sites of Biotransformation • Metabolic enz’s in tissues – Mostly sites of xenobiotic entry • Skin, lung, gut wall – Incr’d levels metab enz’s • Liver significant – Many types of metabolizing enzymes • “Screening organ” – Sees xenobiotics from g.i. – Enterohepatic circulation • Cycles compounds back to liver Toxification/Detoxification • Metab detox’d xenobiotic more easily excr’d • Metab tox’d xenobiotic more harmful to cells, body – Ex: polycyclic aromatic hydroxcarbons epoxidized reactive cmpd Phase I Rxns • Introduce reactive, polar functional grps onto lipophilic mol’s • Modify funct’l grps more hydrophilic • Xenobiotic that looks much diff than parent • Product more easily excr’d OR • Product w/ correct chem. structure to undergo Phase II metab • If not metab’d, lipophilic xenobiotics enter cells or bind serum prot’s & dist’d • Product of Phase I rxns = metabolite more water soluble than parent – More easily excr’d – BUT may be more reactive to cell molecules Redox Review • Reduction/oxidation rxns • Oxidation = loss electrons – Addition O to structure • Ex: epoxidation – Loss H- (H:) – So ox’d cmpds have fewer H’s or more O’s • Reduction = gain electron – Common: gain H– So red’d cmpds have more H’s – Ex: coenzymes (NAD+ NADH) Metabolic Oxidations • Type of Phase I rxn • Frequently by enz’s introducing O – – – – From O2 in body Mixed Function Oxidases (mfo’s) Substr + O2 Prod-OH + H2O Ex: Cytochromes P450 – Impt for endogenous mol’s or nutrients – “Microsomal” • Contained in membr’s of organelles • Sep’d by centrifugation • Key enz’s = Cytochromes P450 – – – – – – Contain heme + Fe + reductase assoc’d Flavin, NAD coenzymes Bind O2, add/receive electrons Liver highest concent in mammals BUT also other tissues Table 3.1 • Not all oxidations are microsomal – Ex: Dehydrogenases oxidize –OH • Fig. 10.3 Metabolic Oxidation Rxns of Carbon • Add –OH grps to C’s of HC’s • Add –O- between 2 C’s w/ multiple bond – If unstable get rearrangement – Epoxide form’n more toxic metabolite • Electron rich • Strained ring structure Metabolic Oxidation Rxns of Noncarbon Elements • N, O, S – Add’n O to N,S • Dehydrohalogenation (nonmicrosomal) • H cleavage near O • Add O Metabolic Reductions • Gen’ly by reductase enz’s – Liver, kidney, lung, others – Intestinal flora enz’s work on S – Reductive dehalogenation Hydrolysis (not a redox rxn) • Add H2O across C-C bond 2 prod’s • Ex: epoxide hydratase • Esters, amides – Impt functional grps hydrolyzed – Found in many pesticides – Esterases, amidases • Found in liver • May detoxify or increase toxicity http://www.blobs.org/science/metabolism/atp/hydrolysis/option2.gif Phase II Reactions • Conjugating – Xenobiotic or metabolite of xenobiotic bound to endogenous cmpd – Endogenous cmpd chem’ly activated yields energy for rxn – Xenobiotic funct’l grp = “chemical handle” to which endogenous cmpd is bound Phase II Reactions • Increases excr’n • Funct’l grp may have been formed by Phase I rxn – Prod more aqueous soluble – Prod less lipid soluble – Prod gen’ly less toxic Phase II Reactions • Glucuronides – Conjugated w/ uridine diphosphate glucuronic acid (UDPGA) – Glucuronyl transferase – Prod’s classified by funct’l grp element to which glucuronide bound • Glutathione (GSH) – Conjugated w/ tripeptide, then further metabolized – Tripeptide = glutamic acid—cysteine—glycine • Cys has –SH to which xenobiotic binds – Further metab mercapturic acid of xenobiotic – Fig. 10.4 – GSH transferase • Several • Specific for diff types chem’s – Glutathione alkyl transferases, epoxide transferases – May enhance toxicity • Final metabolites may bind DNA • Final metabolites may be converted to reactive thiols, bind prot’s/enz’s • Sulfation – Conjugated w/ adenosine-3’-phosphate-5’phosphosulfate (PAPS) – Sulfotransferases – Common substrates: phenols, alcohols, arylamines – Prod’s completely ionized • Very water soluble Modifiers of Biotransformation • Diet – Vitamins, minerals act as coenzymes • Impt to enz function • If missing, decr’d metabolism – Proteins broken down amino acids • Used to make more proteins – Food deprivation changed metab/abs’n toxicants • Hepatic injury – Liver has many biotransforming enzymes – Injury decr’d metab – Diseases • Viral infection (hepatitis) • Jaundice • Cirrhosis • Bioactivation – Metab more reactive agent – Often react w/ nucleophilic sites • Electron-rich • Seek +-charged compounds • -SH, -NH2, -OH • Found on prot’s, nucleic acids Two or More Toxic Substances • Synergism – Total effect greater than sum of individual effects • Potentiation – Inactive substance enhances activity of active substance • Antagonism – Active substance decreases activity of another active substance