* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Biochap2
Protein (nutrient) wikipedia , lookup
Gaseous signaling molecules wikipedia , lookup
Biological aspects of fluorine wikipedia , lookup
Genetic code wikipedia , lookup
Cell-penetrating peptide wikipedia , lookup
Expanded genetic code wikipedia , lookup
Metalloprotein wikipedia , lookup
List of types of proteins wikipedia , lookup
Amino acid synthesis wikipedia , lookup
Proteolysis wikipedia , lookup
Protein adsorption wikipedia , lookup
Fatty acid metabolism wikipedia , lookup
Nucleic acid analogue wikipedia , lookup
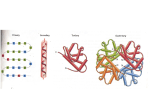
1) Properties of Water Water is a polar covalent solvent •Oxygen end is slightly negative •Hydrogen ends are slightly positive QuickTime™ and a TIFF (Uncompressed) decompressor are needed to see this picture. Hydrogen Bonding • Polarity of water allows attraction between other water molecules – Hydrogen bond - attraction between hydrogen and another highly electronegative atom (FON) – Weaker than covalent or ionic bonds – Water is unique in that one molecule can form up to 4 hydrogen bonds. Properties of Water Cohesion: attraction between 2 molecules of same substance Adhesion: attraction between 2 different molecules -Capillary action - forces between molecules cause water levels to rise against gravity (One way plants draw water from their roots). Solutions and Suspensions • Solution: Type of mixture with components evenly distributed. – Solute: What is dissolved. – Solvent: Substance which solute is dissolved in (usually water). – Like dissolves like! Suspension: Mixture in which material will not dissolve QuickTime™ and a TIFF (Uncompressed) decompressor are needed to see this picture. Acids, Bases, and pH • Water can ionize: H2O H+ + OH- • pH Scales measures the concentration of Hydrogen and Hydroxide ions in solution. – Ranges from 0 to 14 – pH of 7 is neutral (H+ = OH-) – pH less than 7 is considered acidic (H+ > OH-) – pH greater than 7 is called basic (H+ < OH-) – Each value on the pH scale is a a factor of 10! » pH of 3 vs. pH of 5, the pH of 3 has 100 times more hydrogen ions! pH Scale Acids and Bases • Acids are compounds that produce H+ ions in solutions. • Bases are compounds that produce OH- in solution. • *Buffers are weak acids or bases that can react with strong acids/bases to prevent sharp changes in pH. 2) Organic Compounds • Organic Chemistry refers to the study of carbon compounds. – 1) Carbon atoms have 4 valence electrons, and can form 4 individual covalent bonds. – 2) Carbon can bond with itself (single, double or triple covalently), creating the possibility of huge chains. – Polymerization - the formation of large molecules from smaller components. 4 Major Organic Components of Life • 1) Carbohydrates: – Contain C, H, O in a ratio of 1:2:1. – Main source of energy. – Short and long term storage – Name ends in -ose QuickTime™ and a TIFF (Uncompressed) decompressor are needed to see this picture. 1) Carbohydrates – Monosaccharides: single molecule sugars • Glucose, Fructose, Galactose • Chemical Formula C6H12O6 – Disaccharides: double sugars • Maltose, Sucrose, Lactose QuickTime™ and a TIFF (Uncompressed) decompressor are needed to see this picture. QuickTime™ and a TIFF (Uncompressed) decompressor are needed to see this picture. How do macromolecules form? • Dehydration synthesis! – Through a reaction of two molecules and energy, water is produced. – The reverse is known as Hydolysis QuickTime™ and a TIFF (Uncompressed) decompressor are needed to see this picture. Carbohydrates • Polysaccharides: 3 or more simple sugars bonded together. – A) Starch main form of energy storage for plants. – B) Glycogen (Animal starch) form of storage in animals. – C) Cellulose chains of glucose formed in plants for support structures. – D) Chitin is a polysaccharide that makes up exoskeletons of many insects. 2) Lipids - oils, fats, waxes • Contain C, H, O (H:O ratio is greater than 2:1 so lots of Hydrogen!) • Used for long term energy storage (fat tissue) • Make up cell membranes Lipids • Lipids are usually formed through the dehydration synthesis of a glycerol molecule and 3 chains of fatty acids. QuickTime™ and a TIFF (Uncompressed) decompressor are needed to see this picture. Lipids • Saturated is a term that refers to a lipid containing only single covalent bonds between carbon atoms in the fatty acid tails • Unsaturated means that there are one or more double bonds between carbons in the tails of the lipid. QuickTime™ and a TIFF (Uncompressed) decompressor are needed to see this picture. 3) Nucleic Acids • The molecules of heredity! • Contain Hydrogen, Oxygen, Nitrogen, Carbon and Phosphorus. • Individual monomers are known as nucleotides. • Nucleotides have 3 parts: a 5-carbon sugar, a phosphate group, and a nitrogenous base. QuickTime™ and a TIFF (Uncompressed) decompressor are needed to see this picture. Nucleic Acids • There are two possible pentose sugars in a nucleic acid: Ribose and Deoxyribose. • DNA has deoxyribose • RNA has ribose QuickTime™ and a TIFF (Uncompressed) decompressor are needed to see this picture. 4) Protein • The R groups have varying properties: some are acidic, basic, polar, or nonpolar in nature. • Proteins have a diverse role in biology. – Some are for transport (COPI, COPII, actin, myosin) – Structure (muscle, bones) – Regulation of cellular activities (cell cycle) QuickTime™ and a TIFF (Uncompressed) decompressor are needed to see this picture. Protein Organization • 4 levels of organization – Primary = individual sequence of amino acids in a protein. – Secondary = Hydrogen bonding causes folds and twists in between AA (-helix, pleated sheets). – Tertiary = 3-dimensional folding occurs within the chain. – Quaternary = Multiple chains can fold on each other (Hemoglobin). 4) Protein • Proteins contain Carbon, Oxygen, Hydrogen, and Nitrogen. • Building block of protein is called an Amino Acid – AA contain an amino group (-NH2) – Also contain and carboxyl group (-COOH) – 20 different amino acids, each differ only in their side chain (R group).