* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Where is the energy transfer?
Survey
Document related concepts
Transcript
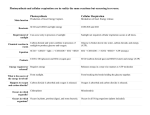
February 14, 2014 Pick up the handout and schedule from the front 1st Law of Thermodynamics The energy of the universe is constant. Energy can be transformed & transferred, but it cannot be created or destroyed. 2nd Law of Thermodynamics Every energy transfer or transformation makes the universe more disordered. In other words, every energy transfer or transformation increases the entropy of the universe Based on free energy changes, reactions can be classified as exergonic (energy outward) or endergonic (energy inward). An exergonic reaction proceeds with a net release of free energy. They occur spontaneously. An endergonic reaction is one that absorbs free energy from its surrounds; the energy is stored in the product; they are nonspontaneous. Energy Transfer Here is a food pyramid that begins with producers and ends with tertiary consumers. If the producer level contains 25,000 kJ of energy and this pyramid follows the 10% rule, then how much energy gets transmitted to the tertiary consumers? 25 kJ All of the questions are printed at the back of your handout Carbon Flow in a Grassland Ecosystem How much carbon (g/m2) is released into the atmosphere as a result of the metabolic activity of the herbivores? 42 g/m2 ΔG = ΔH – TΔS G = Free Energy H = Enthalpy S = Entropy T = Temperature in Kelvin Δ represents change in value over time An experiment determined that when a protein unfolds to its denatured (D) state from the original folded (F) state, the change in Enthalpy is ΔH = H(D) – H(F) = 56,000 joules/mol. Also the change in Entropy is ΔS = S(D) – S(F) = 178 joules/mol. At a temperature of 20⁰C, calculate the change in Free Energy ΔG, in j/mol, when the protein unfolds from its folded state. The correct answer is 3,846 joules/mol. ΔG = ΔH – TΔS ΔG = (56,000 joules/mol) – 293 K (178 joules /mol) ΔG = 56,000 joules/mol – 52,154 joules/mol = 3,846 joules/mol A + B + energy → AB There are many types of biochemical reactions taking place in any living system. Which of the following best characterizes the reaction represented above? A) Catabolism B) Oxidation-reduction C) Exergonic reaction D) Endergonic reaction Energy Transfer When you compare the formula for photosynthesis with cellular respiration, which is exergonic, which is endergonic? They are both oxidation/reduction reactions. 6CO2 + 6H20 + energy C6H12O6 + 6O2 C6H12O6 + 6O26CO2 + 6H20 + energy becomes oxidized C6H12O6 + 6O2 6CO2 + 6H2O + Energy becomes reduced 6CO2 + 6H20 + energy C6H12O6 + 6O2 C6H12O6 + 6O26CO2 + 6H20 + energy CELLULAR RESPIRATION- AN OVERVIEW Where is the energy transfer? Where is the energy transfer? A molecule that is phosphorylated: a.Has an increased chemical reactivity; it is primed to do cellular work b.Has a decreased chemical reactivity; it is less likely to provide energy for cellular work c.Has been oxidized as a result of a redox reaction involving the gain of inorganic phosphate d.Has been reduced as a result of a redox reaction involving the loss of an inorganic phosphate Which of the following statements describes the results of this reaction? C6H12O6 + 6O2 6CO2 + 6H20 + energy a. C6H12O6 is oxidized and O2 is reduced b. O2 is oxidized and H20 is reduced c. CO2 is reduced and O2 is oxidized d. C6H12O6 is reduced and CO2 is oxidized Starting with one molecule of glucose, the “net” products of glycolysis are: a.2 NAD+, 2 H+, 2 pyruvate, 2 ATP and 2 H20 b.2 NADH, 2 H+, 2 pyruvate, 2 ATP, and 2 H20 c.2 FADH2, 2 pyruvate, 4 ATP and 2 H20 d.6 CO2, 6 H20, 2 ATP and 2 pyruvate Where is the energy transfer? Pyruvate (from glycolysis, 2 molecules per glucose) CO2 NAD+ Glycolysis Citric acid cycle ATP ATP Oxidation phosphorylation CoA NADH + H+ Acetyl CoA CoA Where is the energy transfer? CoA Citric acid cycle FADH2 2 CO2 3 NAD+ 3 NADH + 3 H+ FAD ADP + Pi ATP ATP Where is the energy transfer? Where is the energy transfer? Where is the energy transfer? Where is the energy transfer? There is “compartmentalization” within the mitochondrion. What purpose does it serve? How is a concentration gradient important in the process shown here? What is the significance of the inner membrane being folded? Where is the energy transfer? Add it all up- This is probably 26-28 ATP This number is Probably lower The oxygen consumed during cellular respiration is involved directly in which process or event? a.Glycolysis b.Accepting electrons at the end of the end of the electron transport chain c.The citric acid cycle d.The oxidation of pyruvate to acetyl CoA Organic molecules besides carbohydrates can transfer energy Which process in eukaryotic cells will proceed normally whether O2 is present or not and therefore probably evolved first? a.Electron transport b.Glycolysis c.The citric acid cycle d.Oxidative phosphorylation You have a friend who lost 7 kg (about 15 pounds) of fat on a low carb diet. How did the fat leave her body? a. It was released as carbon dioxide and water b. Chemical energy was converted to heat and released c. It was converted to ATP, which weighs less than fat d. It was converted to urine and eliminated from the body Photosynthesis !!! Relate the structure to the functionWe have compartmentalization again Lots of surface area Relate structure and function-At the organ level -At the tissue level -At the cellular level -At the molecular level Photosynthesis- An Overview Where is the energy transfer? Where is the energy transfer? Where is the energy transfer? Where is the energy transfer? C3 Plants Where is the energy transfer? Use these terms to summarize the Calvin Cycle. Carbon dioxide (CO2) Ribulose biphosphate (RuBP) Rubisco 3-phosphoglycerate ATP NADPH Glyceraldehyde-3-phosphate (G3P) Glucose In the Calvin Cycle, CO2 is attached to a molecule of RUBP. This is catalyzed by the enzyme rubisco. The six carbon product splits, forming two molecules of 3-phosphoglycerate. 3-phosphyglycerate receives a phosphate from ATP and electrons from NADPH forming a molecule of G3P. Two molecules of G3P can combine to form a molecule of glucose. Where is the energy transfer? Photosynthetic Adaptations Alternate mode of carbon fixation that forms a 4-carbon compound as its first product. A way to cut down on photorespiration. Sugarcane, corn, members of the grass family (at least 1000 plants) CAM Photosynthesis Evolved in succulent plants, many cacti, pineapples & other plants. Open stomata during night, close during day Compare and contrast the energy transfer in mitochondria & chloroplas Energy Flow in a Hardwood Forest What percentage of the biomass in the forest community is tied up in the Grass layer which has a question mark? The End