* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Plant Metabolism
History of herbalism wikipedia , lookup
Plant stress measurement wikipedia , lookup
Evolutionary history of plants wikipedia , lookup
Venus flytrap wikipedia , lookup
Photosynthesis wikipedia , lookup
History of botany wikipedia , lookup
Historia Plantarum (Theophrastus) wikipedia , lookup
Flowering plant wikipedia , lookup
Ornamental bulbous plant wikipedia , lookup
Plant breeding wikipedia , lookup
Plant evolutionary developmental biology wikipedia , lookup
Plant morphology wikipedia , lookup
Plant nutrition wikipedia , lookup
Plant use of endophytic fungi in defense wikipedia , lookup
Plant reproduction wikipedia , lookup
Plant physiology wikipedia , lookup
Plant defense against herbivory wikipedia , lookup
Plant ecology wikipedia , lookup
Glossary of plant morphology wikipedia , lookup
Sustainable landscaping wikipedia , lookup
Medicinal plants wikipedia , lookup
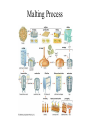
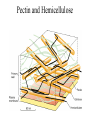
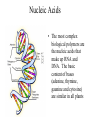
Plant Metabolism Photosynthesis The Most Important Equation in Biology Primary Metabolites • Primary metabolites are compounds that are commonly produced by all plants and that are directly used in plant growth and development. • The main primary metabolites are carbohydrates, proteins, nucleic acids, and lipids. Carbohydrates • Carbohydrates are the sugars made up of glucose and its isomers • Carbohydrates come in many different sizes: • Monosaccharides made up of one sugar unit (glucose or fructose) • Disaccharides made up of two sugar units (sucrose is a glucose and a fructose) • Polysaccharides are polymers made up of more than two sugar units Harvesting Sucrose Sugar Cane Maple Syrup Refining Sucrose Maltose and Sucrose Malting Process Malt Varieties Polysaccharides • Structural polysaccharides are used to support plants • Storage polysaccharides are used to store energy for later use by the plant Structural Polysaccharides • The most common structural polysaccharide in plants is cellulose. It makes up 40 to 60% of the cell wall. It is also the most common polymer on earth • Cellulose is extremely strong due to its chemical organization. It is made of a long chain of beta-glucose molecules – 100 to 15,000 glucose molecules Cotton Boll – Pure Cellulose Gluey Polysaccharides • Pectins are mainly polymers of galacturonic acid. • Hemicelluloses are highly variable and are not related to cellulose. • Grass hemicelluloses are high in xylose, with small amounts of arabinose, galactose, and urionic acids. But pea family (Fabaceae) hemicelluloses are high in arabinose, galactose and urionic acid, but low in xylose. • Some of the most interesting hemicelluloses are not actually used structurally, but rather are exuded from stems, leaves, roots, or fruits in a sticky mixture called a gum Pectin and Hemicellulose Gum Arabic from Acacia senegal Storage Polysaccharides • The most important storage polysaccharides are amylose and amylopectin. Amylose is a long chain of alpha-glucose, several hundred to several thousand molecules long. Amylopectin is more complex, often made up of 50,000 molecules. • These two polymers are both used in making starch grains. Most starch grains are about 20% amylose and 80% amylopectin, but this varies with the plant species. Inulin – another storage carbohydrate – long chain of fructose Jerusalem artichoke Digestability • The properties of alpha-glucose and beta-glucose affect their digestability. Alpha-glucose polymers don’t form fibrils and thus are not as strong as cellulose from betaglucose. Even more important, almost all organisms have alpha-amylase, a digestive enzyme that breaks down alphaglucose bonds. So starches can be digested easily. Plants have alpha-amylase so they can harvest energy from starches in their seeds, roots, and tubers. • Very few organisms have cellulases – just some fungi and bacteria, earthworms, and a few insects can digest cellulase. Proteins • Proteins make up most of the remaining biomass of living plant cells. • A protein consists of one or more polypeptides made up of amino acids. Plants make amino acids from the products of photosynthesis through a very complex process involving the acquisition of N, usually in the form of NH4, and involving the use of large amounts of energy, in the form of ATP and NADPH. Structural Proteins • Structural proteins make up 2 to 10% of the cell wall in plants. Expansins help increase the surface area of cell walls. Extensins help protect or repair damaged cell walls. The plant cell membrane is about 50% structural proteins. Storage Proteins • Storage proteins are used mostly in seeds and are used as source of nutrition for the early development of seedlings. Storage proteins used in seeds vary considerably between plant species. Corn produces a storage protein called ZEIN. Wheat produces a storage protein called GLIADIN Enzymes • Enzymes catalyze biochemical reactions. Most proteins in living cells are enzymes. • Pure enzymes that maintain their activity when removed from plants are commercially important to us. Papaya – Papain and Chymopapain Pineapple - Bromelain Nucleic Acids • The most complex biological polymers are the nucleic acids that make up RNA and DNA. The basic content of bases (adenine, thymine, gaunine and cytosine) are similar in all plants Lipids • Unlike other biological polymers, lipids are not defined by specific, repeating monomeric units. Rather they are defined by their water-repelling properties. The only structure they share is that they mostly are made up of nonpolar hydrocarbon groups (CH3, CH2, and CH). • Oils are fats that are liquid at room temperature. Oils • Oils occur in all parts of a plant, but are most common in seeds. Some seeds have so much oil that it can be commercially harvested. The most commonly used oils are cotton, sesame, safflower, sunflower, olive, coconut, peanut, corn, castor bean, canola, and soybean oils. • The most common seed oil fatty acids are oleic acid (one double bond), linoleic acid (two double bonds), and linolenic acid (three double bonds). Linoleic and linolenic are essential fatty acids – we can’t make them ourselves. Olive Oil Canola Oil Waxes • Waxes are complex mixtures of fatty acids linked to long-chain alcohols. Waxes comprise the outermost layer of leaves, fruits, and herbaceous stems and are called EPICUTICULAR waxes. Waxes embedded in the cuticle of the plant are cuticular waxes. Cutin is another wax in the cuticle and it makes up most of the cuticle. Suberin is a similar wax that is found in cork cells in bark and in plant roots. Both help prevent water loss by the plant. • Structures of waxes vary depending on which plant produced them. • Waxes are usually harder and more water repellant than other fats. Bayberry Wax Euphorbia antisyphilitica Candelilla wax Jojoba Wax Plant Secondary Metabolites • Plants make a variety of less widely distributed compounds such as morphine, caffeine, nicotine, menthol, and rubber. These compounds are the products of secondary metabolism, which is the metabolism of chemicals that occurs irregularly or rarely among plants, and that have no known general metabolic role in plants. • Secondary metabolites or secondary compounds are compounds that are not required for normal growth and development, and are not made through metabolic pathways common to all plants. • Most plants have not been examined for secondary compounds and new compounds are discovered almost daily. Plant Secondary Metabolites • Secondary compounds are grouped into classes based on similar structures, biosynthetic pathways, or the kinds of plants that make them. The largest such classes are the alkaloids, terpenoids, and phenolics. • Secondary compounds often occur in combination with one or more sugars. These combination molecules are known as glycosides. Usually the sugar is a glucose, galactose or rhamnose. But some plants have unique sugars. Apiose sugar is unique to parsley and its close relatives. Functions of Secondary Compounds • The most common roles for secondary compounds in plants are ecological roles that govern interactions between plants and other organisms. • Many secondary compounds are brightly colored pigments like anthocyanin that color flowers red and blue. These attract pollinators and fruit and seed dispersers. • Nicotine and other toxic compounds may protect the plant from herbivores and microbes. • Other secondary compounds like rubber and tetrahydrocannabinil (THC) from cannabis plants have no known function in plants. Alkaloids • Alkaloids generally include alkaline substances that have nitrogen as part of a ring structure. More than 6500 alkaloids are known and are the largest class of secondary compounds. They are very common in certain plant families, especially: • peas – Fabaceae • sunflower – Asteraceae • poppy – Papaveraceae • tomato – Solanaceae • dogbanes – Apocynaceae • milkweeds - Asclepiadaceae • citrus – Rutaceae. Terpenoids • Terpenoids are dimers and polymers of 5 carbon precursors called isoprene units (C5 H8). • Terpenoids often evaporate from plants and contribute to the haze we see on hot sunny days. They are expensive to make; they often take 2% of the carbon fixed in photosynthesis; carbon that could otherwise be used for sugars. Phenolics • Compounds that contain a fully unsaturated six carbon ring linked to an oxygen are called phenolics. • Salicylic acid (basic part of aspirin) is a simple phenol. • Myristicin is a more complex phenol that provides the flavor of nutmeg. • Flavonoids are complex phenolics. They are often sold in health food stores as supplements to vitamin C. The most commonly available flavonoid is rutin from buckwheat. • Anthocyanins are a type of flavonoid that give flowers red and blue pigments. More Phenolics • Some phenolics form polymers. • Tannins are astringent to the taste. They give dryness (astringency) to dry wines. They can also be used to tan leather. They often give water a tea-colored look. Tannins are common in pines and oaks. • Lignin is a major structural component of wood. The exact structure of lignin is complex and not known. Minor Secondary Metabolites • Mustard oil glycosides are nitrogen-sulfur containing compounds that occur in cabbage, broccoli, horseradish, watercress and other members of the mustard family (Brassicaceae). They give the group its characteristic taste and odor. • Cyanogenic glycosides occur in several families of plants, but are especially common in roses (Rosaceae) and peas (Fabaceae). They are sugar containing compounds that release cyanide gas when hydrolyzed. • Cardiac glycosides effect vertebrate heart rate. Especially common in milkweeds Asclepiadaceae. • The parsley/carrot family Apiaceae is noted for having aromatic and poisonous 17 carbon polyacetylenes, though a few species have alkaloids like Coniium. Mustard Oil