* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Nerve activates contraction
Basal metabolic rate wikipedia , lookup
Interactome wikipedia , lookup
Gene expression wikipedia , lookup
Western blot wikipedia , lookup
Artificial gene synthesis wikipedia , lookup
Peptide synthesis wikipedia , lookup
Point mutation wikipedia , lookup
Metalloprotein wikipedia , lookup
Nucleic acid analogue wikipedia , lookup
Protein–protein interaction wikipedia , lookup
Two-hybrid screening wikipedia , lookup
Fatty acid synthesis wikipedia , lookup
Nuclear magnetic resonance spectroscopy of proteins wikipedia , lookup
Genetic code wikipedia , lookup
Amino acid synthesis wikipedia , lookup
Fatty acid metabolism wikipedia , lookup
Proteolysis wikipedia , lookup
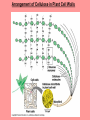
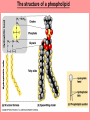
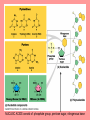
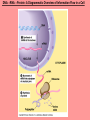
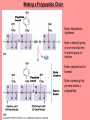
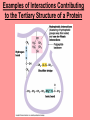
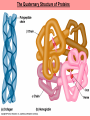
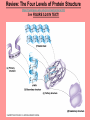
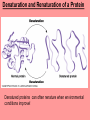
The Structure and Function of Macromolecules Chapter 5 Monomers, Polymers, and Macromolecules • Monomers: repeating units that serve as building blocks for polymers • Polymers: long molecule consisting of many similar or identical building blocks linked by COVALENT bonds • Macromolecules: LARGE groups of polymers covalently bonded – 4 classes of organic macromolecules to be studied: 1. Carbohydrates 3. Proteins 2. Lipids 4. Nucleic Acids Building & Breaking Polymers: http://bcs.whfreeman.com/thelifewire/content/chp03/0302002.html • How do monomers link up to form polymers? – Condensation reaction (specifically, dehydration synthesis): • two molecules covalently bond and lose a water molecule in the process • THIS TAKES ENERGY TO DO! • How do polymers break back into monomers? – Hydrolysis: • polymers are disassembled to monomers by adding a water molecule back • Ex: digestion of food The Synthesis and Breakdown of Polymers As each monomer is added, a water molecule is removed – DEHYDRATION REACTION. This is the reverse of dehydration is HYDROLYSIS…it breaks bonds between monomers by adding water molecules. Organic Compounds and Building Blocks • Carbohydrates – made up of linked monosaccharides • Lipids -- CATEGORY DOES NOT INCLUDE POLYMERS (the grouping is based on insolubility) – Triglycerides (glycerol and 3 fatty acids) – Phospholipids – Steroids • Proteins – made up of amino acids • Nucleic Acids – made up nucleotides CARBOHYDRATES Fuel & Building Material http://bcs.whfreeman.com/thelifewire/content/chp03/0302002.html Carbohydrates – Fuel and Building Material • Carbs include sugars & their polymers • Carbs exist as three types: 1. monosaccharides 2. disaccharides 3. polysaccharides (macromolecule stage) • Made up of C, H, and O in a 1:2:1 ratio (CnH2nOn) • Has carbonyl group (C=O) and multiple hydroxyl groups (-OH) • Size of carbon skeleton determines category The Structure and Classification of Some Monosaccharides REMEMBER: location of carbonyl determines if is an aldose (aldehyde sugar) or a ketose (ketone sugar). See figure 5.3 in text. Monosaccharides • Are major sources of energy for cells! – Ex. Glucose – cellular respiration • Are simple enough to serve as raw materials for synthesis of other small organic molecules such as amino and fatty acids. – Most common: glucose, fructose, galactose Glucose, Fructose, Galactose • Glucose: – made during photosynthesis – main source of energy for plants and animals • Fructose: – found naturally in fruits – is the sweetest of monosaccarides • Galactose: – found in milk – is usually in association with glucose or fructose • All three have SAME MOLECULAR FORMULA but differ structurally so they are ISOMERS! Disaccharides • Consists of two monosaccharides joined by a GLYCOSIDIC LINKAGE – a covalent bond resulting from dehydration synthesis. • Examples: – Maltose – 2 glucoses joined (C12H22O11) – Sucrose – glucose and fructose joined (C12H22O11) – Lactose – glucose and galactose joined (C12H22O11) Examples of Disaccharide Synthesis Polysaccharides • These are the polymers of sugars – the true macromolecules of the carbohydrates. – Serve as storage material that is hydrolyzed as needed in the body or as structural units that support bodies of organisms. These are polymers with a few hundred to a few thousand monosaccharides joined by glycosidic linkages. Storage Polysaccharides – Starch and Glycogen • STARCH AND GLYCOGEN are storage polysaccharides. – Starch: storage for plants – Glycogen: storage for animals Starch • Starch is the storage polysaccharide of PLANTS – made up of glucose monomers in alpha configuration (see fig. 5.7 pg. 67) • Has a helical shape – can be unbranched (amylose) or branched (amylopectin) • Stored as granules in plants in the PLASTIDS – these granules are stockpiles of glucose for later use – carb “BANK”) • You can find starch in potatoes and grains Glycogen • Glycogen is the storage polysaccharide of ANIMALS – extensively branched group of glucose units • Stored in liver and muscle cells • Glycogen bank in humans is depleted within 24 hours and needs replenished by consuming food. Structural Polysaccharides • Cellulose and Chitin are structural polysaccharides: – Cellulose: found in cell wall of PLANTS – Chitin: found in cell wall of FUNGI Cellulose • Major component of plant cell walls – most abundant organic compound on Earth • Cellulose is a polymer of glucose, but all glucose molecules are in the beta configuration – thus, cellulose is always straight, and this provides for strength (Ex. Lumber) Arrangement of Cellulose in Plant Cell Walls Cellulose and the Diet • Few organisms possess the enzymes to digest cellulose – Cellulose passes through the digestive tract and is eliminated in feces • BUT, the fibrils of cellulose abrade the wall of the digestive tract and stimulate secretion of mucus which is necessary for smooth food passage – so though cellulose is not nutritious, it is necessary – Organisms that can digest: cows (with help of bacteria), termites (with help of microbes), some fungi Chitin • Another structural polysaccharide – used by arthropods to build their exoskeletons • Pure chitin is leathery, but when encrusted with calcium carbonate it hardens into shell form • Also used by fungi in their cell walls (instead of cellulose) • Similar to cellulose, but the glucose monomer has a nitrogen containing appendage Chitin, a structural polysaccharide: exoskeleton and surgical thread LIPIDS Energy Storage Lipids http://bcs.whfreeman.com/thelifewire/content/chp03/0302002.html • Does not include polymers – only grouped together based on trait of little or no affinity for water: • Hydrophobic (water fearing) • Hydrophobic nature is based on molecular structure – consist mostly of hydrocarbons! – REMEMBER – hydrocarbons are insoluble in water b/c of their non-polar C—H bonds! Lipids: Highly Varied Group • • • • Smaller than true polymeric macromolecules Insoluble in water, soluble in organic solvents Serve as energy storage molecules Can act as chemical messengers within and between cells • Include waxes and certain pigments – Focus will be on fats, phospholipids, and steroids “Fats” -- Triglycerides • Made of two kinds of smaller molecules – glycerol and fatty acids (one glycerol to three fatty acids) – Dehydration synthesis hooks these up – 3 waters produced for every one triglyceride – ESTER linkages bond glycerol to the fatty acid tails – bond is between a hydroxyl group and a carboxyl group • Glycerol is an alcohol with three carbons, each one with a hydroxyl group • Fatty acid has a long carbon skeleton: – at one end is a carboxyl group (thus the term fatty “acid”) – the rest of the molecule is a long hydrocarbon chain • The hydrocarbon chain is not susceptible to bonding, so water Hbonds to another water and excludes the fats The Synthesis and Structure of a Fat, or Triglycerol • One glycerol & 3 fatty acid molecules • One H2O is removed for each fatty acid joined to glycerol • Result is a fat Saturated vs. Unsaturated “Fats” • Refers to the structure of the hydrocarbon chains of the fatty acids: – No double bonds between the carbon atoms of the chain means that the max # of hydrogen atoms is bonded to the carbon skeleton (saturated) • THESE ARE THE BAD ONES!!! – they can cause atherosclerosis (plaque develop, get less flow of blood, hardening of arteries)! – If one or more double bonds is present, then it is unsaturated • and these tend to kink up and prevent the fats from packing together Examples of Saturated and Unsaturated Fats and Fatty Acids At room temperature, the molecules of a saturated fat are packed closely together, forming a solid. At room temperature, the molecules of an unsaturated fat cannot pack together closely enough to solidify because of the kinks in their fatty acid tails. Fat vs. Oil • Most animal triglycerides are saturated – Ex. Lard, butter – These are solid at room temperature – fat • Plants and fish have unsaturated triglycerides, so they are liquid at room temp – oil – Ex. Vegetable oil, sunflower oil, cod liver oil Saturated and Unsaturated Fats and Fatty Acids: Butter and Oil UNSATURATED SATURATED Are lipids “Bad”? • NO - Major function is energy storage – Ex. Gram of fat stores more than TWICE the energy of a gram of polysaccharide • Since plants are immobile, bulky storage of starch is okay; animals needs mobility, so compact reservoir of fuel (fat or adipose tissue) is better – Adipose tissue provides cushioning for organs and insulation for body Phospholipids • Have only two fatty acid tails! – Third hydroxyl group of glycerol is joined to a phosphate group (negatively charged) • Are ambivalent to water – tails are hydrophobic, heads are hydrophilic. – When added to water, phospholipids self-assemble into aggregates that shield their hydrophobic portions from water: • Ex. micelles – phospholipid droplet with the phosphate head on the outside (figure 5.13) • At cell surface, get a double layer arrangement – phospholipid bilayer The structure of a phospholipid Two structures formed by Self-assembly of Phospholipids in Aqueous Environments Steroids • Characterized by carbon skeleton consisting of four fused rings (see figure 5.14) – Differences depend on the functional groups attached to the ring ensemble • Cholesterol – found in cell membranes of animals, is a precursor from which other steroids may be synthesized – but if is found in high levels in the blood, contributes to atherosclerosis • Many hormones are steroids – Ex: sex hormones Cholesterol: A Steroid • Cholesterol is the molecule from which other steroids, including sex hormones, are synthesized. – Steroids vary in the functional groups attached to their four interconnected rings (shown in gold)… NUCLEIC ACIDS Polymers of Information NUCLEIC ACIDS http://bcs.whfreeman.com/thelifewire/content/chp03/0302002.html POLYMERS OF INFORMATION – BUILDING BLOCKS OF DNA & RNA What Determines the Primary Structure of a Protein? • Gene – unit of inheritance that determines the sequence of amino acids – made of DNA (polymer of nucleic acids) • Building blocks of nucleic acids are nucleotides: – phosphate group, pentose sugar, nitrogenous base (A,T,C,G,U) Two Categories of Nitrogenous Bases • Pyrimidines and Purines: – Pyrimidines: smaller, have a sixmembered ring of carbon and nitrogen atoms (C , U, T) – Purines: larger, have a six- and a fivemembered ring fused together (A, G) NUCLEIC ACIDS consist of: phosphate group, pentose sugar, nitrogenous base Nucleic Acids • Exist as 2 types : DNA and RNA *DNA -- synthesis *RNA -- *double stranded (entire code) *sugar is deoxyribose *never leaves nucleus *bases are A,T,C,G *involved in replication and protein *single stranded (partial code) *sugar is ribose *mobile – nucleus and cytoplasm *bases are A,U,C,G *involved in Protein Synthesis Summary of Flow of Genetic Info • DNA RNA transcription protein translation • Transcription – in nucleus of cell; opens up DNA double helix, copies section needed for protein manufacture, this makes messenger RNA (mRNA) • Translation -- mRNA travels out of nucleus to cytoplasm to a ribosome (site of protein manufacture); ribosomal RNA (rRNA) anchors the transcript in the ribosome, transfer RNA (tRNA) brings in correct amino acid by reading 3 amino acids at a time (codon) DNA→RNA→Protein: A Diagrammatic Overview of Information Flow in a Cell PROTEINS Structural | Storage | Transport | Catalysts Proteins http://bcs.whfreeman.com/thelifewire/content/chp03/0302002.html • Account for over 50% of dry weight of cells • Used for: *structural support (see page 72) *storage *transport *signaling *movement *defense *metabolism regulation (enzymes) • Are the most structurally sophisticated molecules known • Are polymers constructed from 20 different amino acids Hierarchy of Structure • Amino acids – building blocks of proteins – 20 different amino acids in nature • Polypeptides – polymers of amino acids • Protein – one or more polypeptides folded and coiled into specific conformations • All differ in the R-group (also called side chain) • The physical and chemical properties of the R-group determine the characteristics of the amino acid. • Amino acids possess both a carboxyl and amino group. How Amino Acids Join • Carboxyl group of one is adjacent to amino group of another, dehydration synthesis occurs, forms a covalent bond: – PEPTIDE BOND • When repeated over and over, get a polypeptide – On one end is an N-terminus (amino end); – On other is a C-terminus (carboxyl end) Making a Polypeptide Chain Note: dehydration synthesis. Note: carboxyl group of one end attaches to amino group of another. Note: peptide bond is formed. Note: repeating this process builds a polypeptide. Protein’s Function Depends on Its Conformation • Functional proteins consist of one or more polypeptides twisted, folded, and coiled into a unique shape • Amino acid sequence determines shape • 2 big categories – 1. Globular 2. Fibrous Function of a protein depends on its ability to recognize and bind to some other molecule. CONFORMATION IS KEY! Lysozyme Four Levels of Protein Structure 1. Primary Structure: unique sequence of amino acids (long chain) 2. Secondary Structure: segments of polypeptide chain that repeatedly coil or fold in patterns that contribute to overall configuration • are the result of hydrogen bonds at regular intervals along the polypeptide backbone 3. Tertiary Structure: superimposed on secondary structure; irregular contortions from interactions between side chains 4. Quaternary Structure: the overall protein structure that results from the aggregation of the polypeptide subunits The Primary Structure of a Protein This is the unique amino acid sequence…notice carboxyl end and amino end! These are held together by PEPTIDE bonds!!! The Secondary Structure of a Protein Alpha Helix & Beta Pleated Sheet BOTH PATTERNS HERE DEPEND ON HYDROGEN BONDING BETWEEN C=O and N-H groups along the polypeptide backbone. Alpha Helix – delicate coil held together by H-bonding between every fourth amino acid Beta pleated sheet – two or more regions of the polypeptide chain lie parallel to one another. H-bonds form here, and keep the structure together. NOTE – only atoms of backbone are involved, not the amino acid side chains! Tertiary Structure of a Protein • Tertiary structure: superimposed on secondary structure; irregular contortions from interactions between side chains (R-groups) of amino acids: • nonpolar side chains end up in clusters at the core of a protein – caused by the action of water molecules which exclude nonpolar substances • “hydrophobic interaction” • van der Waals interactions, H-bonds, and ionic bonds all add together to stabilize tertiary structure • may also have disulfide bridges form …when amino acids with 2 sulfhydryl groups are brought together – these bonds are much stronger than the weaker interactions mentioned above Examples of Interactions Contributing to the Tertiary Structure of a Protein Quaternary Structure • Quaternary Structure: the overall protein structure that results from the aggregation of the polypeptide subunits – Ex. collagen – structural – Ex. hemoglobin – globular The Quaternary Structure of Proteins Review: The Four Levels of Protein Structure https://mywebspace.wisc.edu/jonovic/web/proteins.html See FIGURE 5.24 IN TEXT! X-ray Crystallography – Figure 5.27 What determines Protein configuration? • Polypeptide chain of given amino acid sequence can spontaneously arrange into 3-D shape – Configuration also depends on physical and chemical conditions of protein’s environment – if pH, salt [ ], temp, etc. are altered, protein may unravel and lose native conformation – DENATURATION •Denatured proteins are biologically inactive! •Anything that disrupts protein bonding can denature a protein! Denaturation and Renaturation of a Protein Denatured proteins can often renature when environmental conditions improve! Protein-Folding Problem • HOW proteins fold is not always clear – may be several intermediate states on the way to stable conformation, but there are a few ways to track, though – – chaperonins: protein molecules that assist the proper folding of other proteins. – computer simulations – “Blue Gene”, a supercomputer able to generate the 3-D structure of any protein starting from its aa sequence (medical uses) A Chaperonin in Action