* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Organic Chemistry
Survey
Document related concepts
Cracking (chemistry) wikipedia , lookup
Hydroformylation wikipedia , lookup
Asymmetric induction wikipedia , lookup
Enantioselective synthesis wikipedia , lookup
Homoaromaticity wikipedia , lookup
Petasis reaction wikipedia , lookup
Organosulfur compounds wikipedia , lookup
Strychnine total synthesis wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Transcript
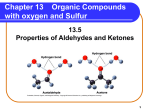
Organic Chemistry Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. 1 Organic chemistry is the study of carbon-containing compounds. Common Elements in Organic Compounds Lack of metals (cations) indicates all covalent bonding SciShow: IDTIMWYTIM “Organic” www.youtube.com/watch?v=9j2a0q14wOs Review: Covalent Bonding Atoms will normally have a number of bonds equal to the number of valence electrons needed. Bonds Formed 4A: 4 x C single bonds 4 3 2 1 1 x C double and 2 single bonds 1 x C triple and 1 single bond 2 x double bonds 5A: 3 x N single bonds 1 x N double and 1 single bond 1 x N triple bond : : 6A: 2 x O single bonds; 1 x O double bond 7A: 1 x F single bond Friedrich Wöhler accidentally synthesized urea in 1828 “When ammonium cyanate is heated, it produces urea without benefit of a kidney, a bladder, or a dog.” • Used biologically for excess nitrogen excretion • First time a biomolecule had been synthetically created • Was believed organics could only be made by living things • Opened gateway to applying chemistry to biological systems Classification of Hydrocarbons Hydrocarbons are made up of mostly hydrogen and carbon. (Contains rings with resonance) Why is Carbon special? Versatility and Stability 1. Versatile bonding: single, double, triple. 2. Forms stable bonds with other Carbon atoms. 3. Forms stable bonds with other non-metal elements. The CAS (Chemical Abstracts Service) Registry contains more than 95 million unique chemical substances on file. Over 80% of them are Organic substances (Carbon compounds). • Small compounds (Acetone, ibuprofen, octane, Vitamin A, ATP) • Biomolecules (Proteins, DNA, Carbohydrates, Lipids) → oil/gas • Plastics (Rubber, PVC, Teflon, Vinyl, Propylene, Styrofoam) Why so many? Carbon is versatile 4 x single bonds Carbon forms stable bonds • Smaller atomic size than Silicon 1 x double and 2 single bonds • Shorter bonds = Stronger bonds 2 x double bonds 1 x triple and 1 single bond • Can bond with a variety of atoms The Petroleum Industry Crude Oil: Complex mixture of 100’s of hydrocarbon variants →“Cracking”: Separate mixture out by boiling point C1-C4 C6-C12 C5-C12 O2 C11-C16 C14-C18 C15-C24 Alkanes – simplest hydrocarbon Alkanes only single covalent bonds • Have the general formula CnH2n+2 where n = 1,2,3,… • Said to be “saturated” because they contain the maximum number of hydrogen atoms that can bond with the number of carbon atoms in the molecule CH4 C2H6 C3H8 methane ethane propane 8 both C4H10 Structural isomers are molecules that have the same molecular formula but different structures. Example 24.1 How many structural isomers can be identified for pentane, C5H12? H H H H H H C C C C C H H H H H H n-pentane (b.p. 36.1 oC) The lack of branching in npentane allows for closer interaction between molecules and stronger intermolecular forces. Physical Properties of Some Alkanes • All alkanes end with “-ane” • Prefix determined by the number of carbon atoms • As mass is added, Melting/Boiling point go up -C1 to C3 are gaseous at room temperature -C4 to C8 are highly volatile liquids 11 Organic Structure Representation • Octonol: C8H18O or C8H17OH • CH3CH2CH2CH2CH2CH2CH2CH2OH • CH3(CH2)7OH • Skeletal Hydrogens are omitted in drawing hydrocarbons and left to be understood (knowing Carbon needs 4 bonds); Non-carbon atoms are explicitly shown • Space-filled Organic Representation Alkane Nomenclature 1. The parent name of the hydrocarbon is that given to the longest continuous chain of carbon atoms in the molecule. CH3 CH3 1 CH2 2 CH2 3 CH 4 4-methylheptane CH2 5 CH2 6 CH3 heptane 7 2. An alkane less one hydrogen atom is an alkyl group. CH4 methane CH3 methyl 14 Alkane Nomenclature 3. Number in the direction that gives the smaller numbers for the locations of the branches. CH3 CH3 CH3 1 CH 2 CH2 3 CH2 4 CH3 5 CH3 CH2 1 2 CH2 3 CH 4 4-methylpentane 1 C 2 CH3 3 CH3 2-methylpentane CH3 CH3 2-methylpentane CH3 5 Alkane Nomenclature 4. Use prefixes di-, tri-, tetra-, when there is more than one alkyl branch of the same kind. CH3 1 CH3 CH3 CH CH 2 3 CH2 4 CH2 5 CH3 6 2,3-dimethylhexane CH3 CH3 1 CH2 2 C 3 CH2 4 CH2 5 CH3 6 CH3 3,3-dimethylhexane 16 Alkane Nomenclature 5. Use previous rules for other types of substituents. CH3 1 Br NO2 CH CH 2 3 CH3 4 2-bromo-3-nitrobutane Cl CH2 1 F CH2 2 CH 3 CH3 4 1-chloro-3-fluorobutane 17 Example Naming Hydrocarbons (nomenclature) Give the IUPAC name of the following compound: The compound is 2,2,4-trimethylhexane. 18 Example Drawing Hydrocarbons from nomenclature Draw the structure of 3-ethyl-2,2-dimethylpentane. Draw the structure of 2-amino-4-isopropyl-5-propyl-octane. 19 Alkane Reactions Combustion CH4 (g) + 2O2 (g) CO2 (g) + 2H2O (l) DH0 = -890.4 kJ/mol Methane (Natural gas) 2C2H6 (g) + 7O2 (g) 4CO2 (g) + 6H2O (l) DH0 = -3119 kJ/mol The greater the number bonds, the greater energy stored. Halogenation CH4 (g) + Cl2 (g) Cl2 + energy light CH3Cl (g) + HCl (g) Cl• + Cl• (Can form radicals – atoms with unpaired e-) Very reactive Hydrocarbon Power! - Crash Course Chemistry #40 www.youtube.com/watch?v=UloIw7dhnlQ Chirality: isomers with identical bonding, but different spatial arrangement. • Enantiomers – non-superimposable isomers with identical mirror images (S & R types). • They appear identical, but are chemically distinct. • Chiral compounds were known to be optically active because they could rotate plane polarized light. • 19 of the 20 amino acids are chiral (L-form) TedEd: What is Chirality?; www.youtube.com/watch?v=71GjsRnsoL8 Optical Isomerism (stereoisomers) No chiral center with 3 unique bonds on C Carbon is a chiral center with 4 unique bonds achiral chiral Alice's Adventures in Wonderland (1865) by Lewis Carroll 23 (S) and (R) Thalidomide Isomers •Introduced in the 1950’s as a “wonder drug” for morning sickness • Resulted in 10,000’s of birth defects • (R) form is effective, but the (S) form can bind to DNA to produce mutagenetic effects. • Many organic reactions produce a racemic mixture of equal parts. Example Chirality Which of the following molecules are chiral? Circle the chiral centers. Chiral Glycine; Achiral Chiral Ibuprofen; Chiral Sold as a racemic mixture Alkenes (unsaturated) Alkenes have the general formula CnH2n where n = 2,3,… • Contain at least one carbon-carbon double bond • Name ends with –ene (or -diene, -triene) CH2 CH CH2 1-butene CH3 CH CH CH2 1,3-butadiene CH2 Geometric Isomers: Diastereomers Cis: Larger R’s on same side Cl Cl C H Cl C C H cis-dichloroethylene H Trans: Larger R’s on opposite side H C Cl trans-dichloroethylene Cis-isomers Trans-isomers Geometric Isomers: Diastereomers cis-1,2-dichloroethene trans-1,2-dichloroethene cis-butenedioic acid (maleic acid) trans-butenedioic acid (fumaric acid) Oleic acid (~70% olive oil) Elaidic acid *Trans-fats are considered worse nutritionally because they pack tightly (more difficult to break apart as they stick together) Less branching leads to stronger London Dispersion forces (e.g. higher melting point) Cis-Trans Isomerization in the Vision Process Electron micrograph of retinal rods 28 Example Give the IUPAC name of the following Alkenes: • Counting carbons starts at 1st double bond (not 1st substituent) NH2 Br C H 1 C CH2 CH3 4 H2C CH CH2 CH2 CH2 H 1-bromo-cis-1-butene 3-amino-1,4-pentadiene Neither cis or trans 29 Alkene Reactions Cracking (breaking down to smaller hydrocarbons) C2H6 (g) Pt catalyst CH2 CH2 (g) + H2 (g) Addition Reactions CH2 CH2 (g) + HBr (g) (Oxidation: lose H’s) CH3 CH2Br (g) (Reduction: gain H’s) CH2 CH2 (g) + Br2 (g) CH2Br CH2Br (g) d+ d- and/or Markovnikov’s rule: When adding polar reagents to alkenes, the positive portion (d+) of the reagent (usually hydrogen) adds to the carbon atom that already has the most hydrogen atoms. 31 Alkynes (Triple bonds) Alkynes have the general formula CnH2n-2 where n = 2,3,4,… • Contain at least one carbon-carbon triple bond • Name ends with –yne CH C CH2 CH3 CH3 1-butyne C C CH3 2-butyne (very rare in biochemistry) Production of acetylene CaC2 (s) + 2H2O (l) C2H2 (g) + Ca(OH)2 (aq) Crash Course: Alkenes & Alkynes www.youtube.com/watch?v=CEH3O6l1pbw 32 Cycloalkanes Alkanes with carbon atoms joined in rings. • They have the general formula CnH2n • Each ring adds a degree of unsaturation (2 less H per ring) • Begin with the prefix Cyclo- 33 H H H C C C C C C Aromatic Hydrocarbons H H C H H benzene C C C C H H H Phenol H C H TNT Aspirin Electron micrograph of benzene Benzene compounds were originally coined Aromatic due to being commonly volatile with distinct odors (aroma). While aromatic may often have smells, there is no firm rule stating one must trigger an olfactory response. Strawberry Vanillin Naphthalene (moth balls) Eugenol (Carnations) Guaiacol (roasted coffee) (Bacon) Aromatic rings have resonance (delocalized electrons) across their p-bonds: increases stability • Benzene (benzyl) when main component • Phenyl group when a substituent Nitrobenzene 3 of the 20 amino acids possess aromatic groups trans-2-phenyl-2-butene Crash Course: Aromatic Compounds www.youtube.com/watch?v=kXFEex-dABU Chemistry In Action: Ice That Burns methane hydrate ~1013 tons of carbon content Found on ocean sea-bed Difficult to harness energy 37 Functional Groups Specific groups of atoms or bonds within molecules that are responsible for the molecules’ characteristic properties Functional Group Chemistry Alcohols contain the hydroxyl functional group (R−OH). • Form Hydrogen bonds • Not Acidic; does not deprotonate • 3 of the 20 amino acids possess –OH • Nomenclature suffix: –nol Drinking Alcohol Rubbing Alcohol Wood Alcohol “AntiFreeze” BPA ← Oxalic Acid - Kidney Ethanol Production and Metabolism Biological production of ethanol Enzymes C6H12O6 (aq) Sugar 2CH3CH2OH (aq) + 2CO2 (g) “Fermentation” Commercial production of ethanol CH2 CH2 (g) + H2O (g) H2SO4 Metabolism of ethanol CH3CH2OH CH3CH2OH (g) Acetaldehyde alcohol dehydrogenase CH3CHO + H2 “Hangover molecule” Acetylaldehyde dehydrogenase enzyme O Disulfiram inhibitor “Antabuse” H C CH3 Acetaldehyde O H C OH + H2 Acetic Acid Functional Group Chemistry Ethers have the general formula R−O−R′. Diethyl ether: solvent Lignin: found in cell wall of plants Polysaccharides: etherlinkage between sugars Polyethylene glycol: Common polymer in pharmaceuticals, cosmetics, and commercial use. crown ether Functional Group Chemistry Aldehydes and Ketones contain the Carbonyl group. O • Aldehydes have the general formula R C H • Nomenclature suffix: -al O H C H Formaldehyde Cinnamaldehyde (2E)-3-phenylprop-2-enal (Methanal; Embalming Fluid) O H CH3 C CH3 Acetyl Acetaldehyde (Ethanal) O • Ketones have the general formula R C R′ • Nomenclature suffix: -one Fructose O H3C C CH3 acetone Acetophenone (1-phenylethanone) (2-propanone, Nail polish remover) Functional Group Chemistry Carboxylic acids contain the carboxyl ( −COOH ) group. • A Carboxyl group is a carbonyl next to a hydroxyl group • All amino acids have a carboxylic acid used in peptide linking. • Have low pKa and deprotonate easily. • Nomenclature suffix: –ic acid Fatty acids CH3(CH2)nCOOH Esters have the general formula R′COOR Ester-linkages in lipids • Formed from reaction of alcohol & carboxylic acid • More easily broken than ethers • Nomenclature suffix: -oate Esters have characteristic fruity aromas Strawberry Oranges Benzyl acetate Octyl acetate Grapes Methyl anthranilate Apples Bananas Pentyl pentanoate Pentyl acetate Functional Group Chemistry Amines are organic bases with the general formula R3N. • R-NH2 + H+ → R-NH3+ (can be neutral or positively charged) • All amino acids have an amine at their base Primary amine Secondary amine Tertiary amine +NH 3 Glutamine Porphyrin ring: region in Hemoglobin bound to Fe+2 used to bind O2 (both 2° and 3° amines) Functional Group Chemistry Amides possess a carbonyl adjacent to an amine. • NOT Basic; does NOT accept H+ to become + charged • Very stable bond due to resonance +NH 3 Glutamine Amines react with Carboxylic acids to form Amide bonds • Called a peptide bond in proteins when linking amino acids Functional Group Chemistry Thiols possess Sulfur atoms •Similar bonding as oxygen, but weaker bonds •Notable odor resembling garlic or rotten eggs Vitamin B1: Thiamine Thioacetone S C H3C CH3 SciShow: 5 of the World's Most Dangerous Chemicals www.youtube.com/watch?v=ckSoDW2-wrc Disulfide Bridges Spot the Functional Groups in the molecules Acetoacetic acid Cinnamaldehyde Heme A Maltose Amine (amino) Amide Alcohol (hydroxyl) Aldehyde Aromatic (Phenyl) Ester Ether Carboxylic Acid (Carboxyl) Ketone (Carbonyl) Thiol Spot the Nine Functional Groups in the molecule: C18H12N2O9 (2Z)-5-[5-(acetyloxy)-3-(2-amino-2-oxoethyl)-2-hydroxyphenyl]-4-amino-5-oxo-2-[(2-oxoethoxy)methyl]pentanoic acid Crash Course: Functional Groups; www.youtube.com/watch?v=hlXc_eEtBHA Crash Course: Organic Nomenclature; www.youtube.com/watch?v=U7wavimfNFE Other Notable groups Steroids: Purines: Pyrimidines: Testosterone Adenine Cytosine Cholesterol Caffeine Barbiturates Reactions: The Science of Caffeine www.youtube.com/watch?v=YuJOhpNS0IY Ribose: Found in DNA/RNA ATP *NADH Organic Reactions • Heterolytic – polar reactions – More common reaction in biological reactions – Bonding e- pair go with 1 atom : • Homolytic – radical reactions – Less specific – Forms free radicals by atoms splitting the e- pair Heterolytic (Polar) Reactions • Occur between attraction of partial + & – charges on different functional group atoms in molecules • Electron-rich atom react with electron-poor atoms. – Nucleophile – electron rich (lone pair; d-) and “nucleusloving”. Donate electron pair to form bond. – Electrophile – electron poor (d+) and “electron-loving”. Polarity/Stability The more polarized a bond is the more prone it is to react. d- d+ d+ d+ d- d• Added Carbonyl increases C─O bond polarity. • C─O bond is more easily broken in esters over ethers. Condensation and Hydrolysis reactions + H2O Alcohol Acid Acid Ester 2 Alcohols to ether Amine Amide Homolytic (Radical) Reactions Initiator: Cl2 + UV light → 2Cl• Ethylene Will continue to add ethylene units until it ethylene runs out and reacts with another radical to cease radical reaction. repeating unit (monomer); n can equal 100’s to 100,000’s Polyethylene Plastic A polymer is a large compound made up of many repeating chemical units. (poly = many; mer = unit) Natural polymers Synthetic polymers (plastics) • Proteins – Amino acid • Polyethylene • DNA – Nucleic acid base •Polypropylene • Cellulose - Carbohydrates • Polyvinlylchloride (PVC) • Rubber - Isoprene • Nylon • Teflon • Kevlar Proteins bound to DNA Teflon: polytetrafluoroethylene Changing the monomer composition changes the properties and type of plastic. Polypropylene Propylene Polyvinyl chloride (PVC) Chloro-ethylene Polystyrene (Styrofoam) Polytetrafluoroethylene (Teflon) Copolymers possess more than one type of monomer Acrylonitrile butadiene rubber Styrene-butadiene rubber Crash Course: Polymers www.youtube.com/watch?v=rHxxLYzJ8Sw http://www.compoundchem.com/2014/06/22/kevlar/ Chemical Identification: IR Spectroscopy Covalent bonds have various motions that have infrared frequencies C=C Bond Bending 61 Chemical Identification: 13C NMR 13C nuclei align in a magnetic field; a pulse of a precise frequency can cause them to flip alignment; dependent on local chemistry Alcohol C13-O-H Vanillin Ether C13-O-C13 Aromatic; C=C Aldehyde (13C=O)H Chemical Identification: 1H NMR Much like 13C NMR, 1H orients in a magnetic field and is flipped by a specific frequency that is dependent on local chemistry. Ether 1H-C-O-C Vanillin Aromatic; Alcohol C-O-1H C=C Aldehyde (C=O)1H