* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Carbon Chemistry
Survey
Document related concepts
Transcript
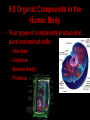
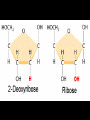
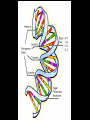
Bellwork 3/6/09 • What is a covalent bond? • What is an ionic bond? • Give an example of each. Carbon Chemistry 9.1 Carbon Compounds • Organic compounds – are compounds that contain carbon and hydrogen, often combined with a few other elements such as oxygen and nitrogen. • 90% of all known compounds Forms of Carbon • Diamond – Hardest substance on Earth! – Is a network solid, all the atoms are linked by covalent bonds. It is a single molecule. – Covalent bonds connect each carbon to four other carbon atoms. • Graphite – Extremely soft and slippery. – Widely spaced layers – In each layer, each carbon atom forms strong covalent bonds with three other carbon atoms. – Between each layer bonds are weak. – Pencil Lead • Fullerenes – Large hollow spheres. – Found in meteorites. – Alternating hexagons and pentagons. – 60 carbon atoms Saturated Hydorcarbons • Hydrocarbon – an organic compound that contains only hydrogen and carbon. • Saturated hydrocarbon – all of the bonds are single bonds. – Maximum number of hydrogens for each carbon. – Other name is Alkane. • Straight Chain – Molecular formulas show the type and number of atoms in a molecule. • Branched Chains – Compounds with the same molecular formula but different structural formulas are isomers. • Rings – Called ring alkanes or cyclo -. Unsaturated Hydrocarbons • a hydrocarbon that contains one or more double or triple bonds. • There are three types of unsaturated hydrocarbons – alkenes, alkynes, and aromatic hydrocarbons Bellwork – 3/9/09 254 – 71 92 + 54 452 + 211 Mr. Thomas, Bellwork - What are the three forms of Carbon? Describe each form. 9.2 Substituted Hydrocarbons • A hydrocarbon in which one or more hydrogen atoms have been replaced by an atom or group of atoms is a substituted hydrocarbon. • The substituted atom or group of atoms is called a functional group because it determines the properties or function of the compound. • Alcohol – The functional group in an alcohol is a hydroxyl group, -OH. • Organic Acids – The functional group in organic acids is a carboxyl group, -COOH – Organic acids have sharp tastes and strong odors. (Vinegar) • Organic Base – Amines are organic bases. – The functional group in an amine is an amino group, -NH2. – Amines play a vital role in life. • Esters – The functional group of an ester is an inorganic or organic acid. – Account for flavors of many foods and pleasant aroma of many flowers. Butyl butyrate pineapple Methyl phenylacetate honey Ethyl nail polish remover, model paint, acetate model airplane glue Ethyl lactate butter, cream Bellwork 209 x 4 150 x 7 56 x 5 An unknown compound has no noticeable odor. Explain why the compound is unlikely to be an organic acid, an organic base, or an ester. 9.3 Organic Compounds in the Human Body • Four types of compounds produced in plant and animal cells: – Starches – Cellulose – Nucleic Acids – Proteins Starches • • • • AKA – Sugars Simple sugar formula – C6H12O6 Exist as straight chains or rings Carbohydrates are made up of simple sugars, more complex sugars, and polymers. Proteins • Organic Acids (-COOH)/Organic Bases (-NH2) • An amino acid is a compound that contains both a carboxyl and amino functional groups in the same molecule. • There are 20 amino acids that your body needs to function. • Your cells can manufacture some, but not all, of the amino acids. • Your cells use amino acids, to form polymers called Proteins. • A protein is a polymer in which at least 100 amino acids are linked through bonds between an amino group and a carboxyl group. • The instructions for building proteins are stored in your DNA. Nucleic Acids • Store information about cell structure and function. • Nucleic acids are large nitrogen containing polymers found mainly in the nuclei of cells. • Two types: Deoxyribonucleic Acid (DNA) Ribonucleic Acid (RNA) • Nucleic Acids are made up of monomers called nucleotides.