* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Slide 1
Survey
Document related concepts
Transcript
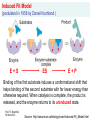
CP504 – ppt_Set 02 Enzyme kinetics and associated reactor design: Introduction to enzymes, enzyme catalyzed reactions and simple enzyme kinetics - learn about enzymes - learn about enzyme catalyzed reactions - study the kinetics of simple enzyme catalyzed reactions Prof. R. Shanthini 09 Nov 2012 What is an Enzyme? Enzymes are mostly proteins, and hence they consists of amino acids. Enzymes are present in all living cells, where they help converting nutrients into energy and fresh cell material. Enzymes breakdown of food materials into simpler compounds. Examples: - pepsin, trypsin and peptidases break down proteins into amino acids - lipases split fats into glycerol and fatty acids - amylases break down starch into simple sugars Prof. R. Shanthini 09 Nov 2012 What is an Enzyme? Enzymes are very efficient (biological) catalysts. Enzyme catalytic function is very specific and effective. Enzymes bind temporarily to one or more of the reactants of the reaction they catalyze. Enzymes does not get consumed in the reaction that it catalyses. Prof. R. Shanthini 09 Nov 2012 How does an Enzyme help? Enzymes speed up reactions enormously. To understand how they do this, examine the concepts of activation energy & the transition state. In order to react, the molecules involved are distorted, strained or forced to have an unlikely electronic arrangement. That is the molecules must pass through a high energy state. This high energy state is called the transition state. The energy required to achieve it is called the activation energy for the reaction. Prof. R. Shanthini 09 Nov 2012 How does an Enzyme help? The higher the free energy change for the transition barrier, the slower the reaction rate. Prof. R. Shanthini 09 Nov 2012 How does an Enzyme help? Enzymes lower energy barrier by forcing the reacting molecules through a different transition state. This transition state involves interactions with the enzyme. Enzyme Prof. R. Shanthini 09 Nov 2012 Enzyme classification Oxidoreductase: transfer oxygen atoms or electron Transferase: transfer a group (amine, phosphate, aldehyde, oxo, sulphur, etc) Hydrolase: hydrolysis Lyase: transfer non-hydrolytic group from substrate Isomerase: isomerazion reactions Ligase: bonds synthesis, using energy from ATPs Prof. R. Shanthini 09 Nov 2012 Examples Examples of of enzyme Enzymecatalyzed Catalysedreactions Reactions Example 1: CO2+ H2O Carbonic anhydrase H2CO3 Carbonic anhydrase is found in red blood cells. It catalyzes the above reaction enabling red blood cells to transport carbon dioxide from the tissues (high CO2) to the lungs (low CO2). One molecule of carbonic anhydrase can process millions of molecules of CO2 per second. Prof. R. Shanthini 09 Nov 2012 Examples of enzyme catalyzed reactions Example 2: 2H2O2 Catalase 2H2O + O2 Catalase is found abundantly in the liver and in the red blood cells. One molecule of catalase can breakdown millions of molecules of hydrogen peroxide per second. Hydrogen peroxide is a by-product of many normal metabolic processes. It is a powerful oxidizing agent and is potentially damaging to cells which must be quickly converted into less dangerous substances. Prof. R. Shanthini 09 Nov 2012 Industrial use of catalase - in the food industry for removing hydrogen peroxide from milk prior to cheese production - in food-wrappers to prevent food from oxidizing - in the textile industry to remove hydrogen peroxide from fabrics to make sure the material is peroxide-free - to decompose the hydrogen peroxide which is used (in some cases) to disinfect the contact lens Prof. R. Shanthini 09 Nov 2012 Examples of Industrial Enzymes See the hand out on the same topic Prof. R. Shanthini 09 Nov 2012 More on enzymes Enzymes are very specific. Absolute specificity - the enzyme will catalyze only one reaction Group specificity - the enzyme will act only on molecules that have specific functional groups, such as amino, phosphate or methyl groups Linkage specificity - the enzyme will act on a particular type of chemical bond regardless of the rest of the molecular structure Stereochemical specificity - the enzyme will act on a particular steric or optical isomer Prof. R. Shanthini 09 Nov 2012 Prof. R. Shanthini 09 Nov 2012 Source: http://waynesword.palomar.edu/molecu1.htm E+S Prof. R. Shanthini 09 Nov 2012 ES Source: http://waynesword.palomar.edu/molecu1.htm Lock & Key Theory Of Enzyme Specificity (postulated in 1894 by Emil Fischer) E+S Prof. R. Shanthini 09 Nov 2012 ES E+P Source: http://waynesword.palomar.edu/molecu1.htm Prof. R. Shanthini 09 Nov 2012 Active Site Of Enzyme Blocked By Poison Molecule Prof. R. Shanthini 09 Nov 2012 Source: http://waynesword.palomar.edu/molecu1.htm Induced Fit Model (postulated in 1958 by Daniel Koshland ) E+S ES E+P Binding of the first substrate induces a conformational shift that helps binding of the second substrate with far lower energy than otherwise required. When catalysis is complete, the product is released, and the enzyme returns to its uninduced state. Prof. R. Shanthini 09 Nov 2012 Source: http://www.mun.ca/biology/scarr/Induced-Fit_Model.html Simple Enzyme Kinetics E+S k1 k3 ES E+P k2 which is equivalent to [E] S Prof. R. Shanthini 09 Nov 2012 P S for substrate (reactant) E for enzyme ES for enzyme-substrate complex P for product Michaelis-Menten approach to the rate equation: E+S k1 ES k3 E+P k2 Assumptions: 1. Product releasing step is slower and it determines the reaction rate 2. ES forming reaction is at equilibrium 3. Conservation of mass (CE0 = CE + CES) Initial concentration of E Concentration of E at time t Prof. R. Shanthini 09 Nov 2012 Concentration of ES at time t Michaelis-Menten approach to the rate equation: E+S k1 ES k3 E+P k2 Product formation (= substrate utilization) rate: rP = - rS = k3 CES (1) Since ES forming reaction is at equilibrium, we get k1 CE CS = k2 CES Prof. R. Shanthini 09 Nov 2012 (2) Michaelis-Menten approach to the rate equation: E+S k1 ES k3 E+P k2 Using CE0 = CE + CES in (2) to eliminate CE, we get k1 (CE0 – CES) CS = k2 CES which is rearranged to give CES = Prof. R. Shanthini 09 Nov 2012 CE0CS k2/k1 + CS (3) Michaelis-Menten approach to the rate equation: E+S k1 k3 ES E+P k2 Using (3) in (1), we get rP = - rS = k3CE0CS k2/k1 + CS = rmaxCS KM + CS where rmax = k3CE0 (5) and KM = k2 / k1 (6) Prof. R. Shanthini 09 Nov 2012 (4) Other terminology used Catalytic step E+S k1 k3 ES E+P k2 Substrate binding step k3 = kcat rmax = k3CE0 = kcatCE0 KM = k2 / k1 Prof. R. Shanthini 09 Nov 2012 (6) (5a) Briggs-Haldane approach to the rate equation: E+S k1 ES k3 E+P k2 Assumptions: 1. Steady-state of the intermediate complex ES 2. Conservation of mass (CE0 = CE + CES) Initial concentration of E Concentration of E at time t Concentration of ES at time t Prof. R. Shanthini 09 Nov 2012 Briggs-Haldane approach to the rate equation: E+S k1 ES k3 E+P k2 Product formation rate: rP = k3 CES (7) Substrate utilization rate: rs = - k1 CECS + k2 CES (8) Since steady-state of the intermediate complex ES is assumed, we get k1 CECS = k2 CES + k3 CES Prof. R. Shanthini 09 Nov 2012 (9) Briggs-Haldane approach to the rate equation: E+S k1 ES k3 E+P k2 Combining (7), (8) and (9), we get rP = - rS = k3 CES (10) Using CE0 = CE + CES in (9) to eliminate CE, we get k1 (CE0 - CES)CS = (k2 + k3)CES which is rearranged to give CES = Prof. R. Shanthini 09 Nov 2012 CE0CS (k2+k3)/k1 + CS (11) Briggs-Haldane approach to the rate equation: E+S k1 k3 ES E+P k2 Combining (10) and (11), we get rP = - rS = k3CE0CS (k2+k3)/k1 +CS where rmax = k3CE0 and KM = (k2 + k3) / k1 = rmaxCS KM + CS (5) (13) When k3 << k2 (i.e. product forming step is slow), Prof. R. Shanthini 09 Nov 2012 KM = k2 / k1 (6) (12) Simple Enzyme Kinetics (in summary) [E] S rP = - r S = P rmaxCS KM + CS where rmax = k3CE0 = kcatCE0 and KM = f(rate constants) rmax is proportional to the initial concentration of the enzyme KM is a constant Prof. R. Shanthini 09 Nov 2012