* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Antidementia
Atypical antipsychotic wikipedia , lookup
Orphan drug wikipedia , lookup
Drug interaction wikipedia , lookup
Clinical trial wikipedia , lookup
Pharmacognosy wikipedia , lookup
Prescription costs wikipedia , lookup
Pharmaceutical industry wikipedia , lookup
Nicotinic agonist wikipedia , lookup
Theralizumab wikipedia , lookup
Neuropharmacology wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
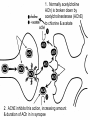
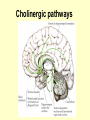
Anti Dementia drugs Anti-dementia drugs • Cholinesterase Inhibitors (aka anticholinesterase drugs, cholinergic agents). • Complementary medicines • Vitamins • Antipsychotics for treatment of psychotic symptoms & or agitation/aggression. Cholinesterase Inhibitors • • • • • Tacrine (Cognex) - Now seldom prescribed Donepezil (Aricept)* Galantamine hydrobromide (Reminyl)* Rivastigmine (Exelon)* *TGA approval, now on PBS for mild to moderate Dementia of the Alzheimer’s Type (DAT) • Stabilise decline. • Do not halt or reverse disease. Action • DAT is characterised by the loss of the brain neurotransmitter, acetylcholine. • Anti-dementia drugs act by increasing brain levels of acetylcholine via blockade of the enzyme that normally breaks it down. 1. Normally acetylcholine ACh) is broken down by acetylcholinesterase (AChE) to chlorine & acetate 2. AChE inhibits this action, increasing amount & duration of ACh in in synapse Rivastigmine (Exelon). Novartis Donepezil (Aricept). Pfizer Galantamine Reminyl (Janssen-Cilag) Cholinergic pathways PBS requirements • Step 1 • A diagnosis of probable Alzhiemer’s must be confirmed by a specialist (geriatrician or psychiatrist) • Step 2 • Mini Mental State Examination (MMSE) is performed. Step 3 • If MMSE score 10-24, approval is granted for 1 months supply with up to 5 repeats. • If MMSE is > 24 it is also necessary to perform a baseline Alzhiemer’s Disease Assessment Scales, Cognitive sub-scale (ADAS-Cog) or referral to a specialist memory disorders unit. The results must accompany the application to prescribe. Step 4 • For continued authority to prescribe it is necessary to demonstrate an improvement in MMSE score of at least 2 points from baseline. • For patients >24, a decrease in the ADAS-Cog score of 4 points or greater is required. • The optimal time to perform tests is 4-8 weeks after maximum dose achieved. Summary of trials • • • • • Only modest improvement overall. Greatest improvement with higher doses. Higher doses less well tolerated. Long term efficacy unknown. Clinical effectiveness in severe disease has not been demonstrated. • Only mildly or moderately affected individuals were selected for trials Side Effects • • • • • Dose related Cholinergic effects: diarrhoea nausea vomiting anorexia & weight loss Minimize side effects by: Start low go slow. Complimentary • Ginkgo Biloba • 52 week, double blind, placebo controlled trial. N=309. • Mild to mod. DAT & Multi infarct dementia. • Small benefits vs placebo. • Well tolerated • High drop out rate. (Ernst & Pittler, 1999, Le Bars, 1997) Selegiline & Vitamin E • Each agent delayed progression to moderate to severe dementia, loss of basic ADL’s, nursing home placement or death. • Less benefit with combined therapy. • Vit E delayed nursing home placement by 230 days compared to placebo. (Sano et al, 1997) References Ernst, E., & Pittler, M. H. (1999). Ginkgo biloba for dementia - A systematic review of double-blind, placebo-controlled trials. Clinical Drug Investigation, 17(4), 301-308. Lebars, P. L., Katz, M. M., Berman, N., Itil, T. M., Freedman, A. M., & Schatzberg, A. F. (1997). A Placebo-Controlled, Double-Blind, Randomized Trial of an Extract of Ginkgo Biloba for Dementia. Jama: Journal of the American Medical Association, 278(16), 1327-1332. References National Prescribing Service. (2001). New alzheimer's drugs show only modest benefits. National Prescribing Service Newsletter, June 01. pdf References Sano, M., Ernesto, C., Thomas, R. G., Klauber, M. R., Schafer, K., Grundman, M., Woodbury, P., Growdon, J., Cotman, D. W., Pfeiffer, E., Schneider, L. S., & Thal, L. J. (1997). A Controlled Trial of Selegiline, AlphaTocopherol, or Both as Treatment for Alzheimers Disease. New England Journal of Medicine, 336(17), 1216-1222.