* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Directive 2010/84/EU - Association of Pharmacy Technicians, UK
Pharmaceutical marketing wikipedia , lookup
Psychedelic therapy wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Drug design wikipedia , lookup
Neuropharmacology wikipedia , lookup
Adherence (medicine) wikipedia , lookup
Electronic prescribing wikipedia , lookup
Zoopharmacognosy wikipedia , lookup
Pharmacokinetics wikipedia , lookup
Compounding wikipedia , lookup
Pharmaceutical industry wikipedia , lookup
Prescription drug prices in the United States wikipedia , lookup
Drug discovery wikipedia , lookup
Prescription costs wikipedia , lookup
Pharmacogenomics wikipedia , lookup
Theralizumab wikipedia , lookup
Drug interaction wikipedia , lookup
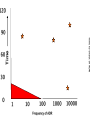
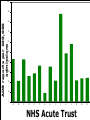
Yellow Card Reporting and what it means for Pharmacy Technicians Dr Anthony R Cox Yellow Card Centre West Midlands Benefits ADR Definition Directive 2010/84/EU A response to a medicinal product which is noxious and unintended and which occurs at doses normally used in man for the prophylaxis, diagnosis, or therapy of disease or for the restoration, correction, or modification of physiological function. Directive 2010/84/EU New to ADR definition: “not only from the authorised use of a medicinal product at normal doses, but also from medication errors and uses outside of the marketing authorisation, including misuse and abuse of the medicinal product.” Admissions In-patients 1955 neurological reports passed to company, 1960 Company stated “non toxic” 1961 withdrawn, 90,000 miscarriages, thousands deformed Lessons Need for adequate testing Need for regulation Need for pharmacovigilance systems (spontaneous reporting) Avoidance of unnecessary use of drugs in pregnancy Some risks cannot be minimised 1 The Black Symbol – article 23 list of medicinal products that are subject to additional monitoring – All medicines with a new active substance and all biologicals – Medicines which require further information after authorisation – Medicines subject to conditions or restrictions on safe and effective use Black Symbol (Triangle) “This medicinal product is subject to additional monitoring" The mean number of people in phase three trials for an NSAID in Europe, in 1994, was 2128 patients Homma. Drug Inj J 1994 . Time Patient Reporting • Different types of reports • New signals “Pharmacists lack the knowledge of clinical medicine necessary to recognise adverse drug reactions. However, their knowledge of pharmacology and toxicology should ensure a role for them in the prediction and prevention of adverse drug reactions” 1986 Some ADRs are easy to spot… Where do reports come from F R A N C I S 100 90 ADR reports per 100,000 admissions 80 70 60 50 40 30 20 10 0 A B C D E F G H I J K L M NHS Acute Trust N O Pharmacy should be the main source of Yellow Card Reports. What is stopping us? Email: [email protected]