* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download the Lesson!
Membrane potential wikipedia , lookup
Biochemistry wikipedia , lookup
Western blot wikipedia , lookup
Lipid bilayer wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Signal transduction wikipedia , lookup
Endomembrane system wikipedia , lookup
Cell membrane wikipedia , lookup
Model lipid bilayer wikipedia , lookup
Cell-penetrating peptide wikipedia , lookup
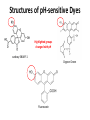
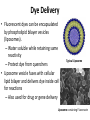
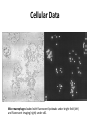
Fluoresecent pH-dependent Lipobeads in vivo pH changes in biology • pH changes are important to several biological processes: • muscle contraction, endocytosis, cell proliferation, apoptosis, ion transport • Biological enzymes function at an optimal pH • Folded protein is stabilized by the specific ions • Protein enzyme ATPase uses a proton gradient for energy production Standard pH meter • pH electrode – glass electrode filled with electrolyte and Ag/AgCl reference electrode – thin glass membrane in contact with solution – Potential difference builds up over the thin glass membrane due to differences in H+ concentration – Potential measured against reference electrodes and pH calculated • Too big to be implanted in a cell! Fluorescence Non-radiative relaxation Vibration relaxation Fluorescence 1. Absorption • excites the molecule to excited state • some molecules may be in vibrationally or rotationally excited states 2. Vibrational relaxation • molecule transitions to lowest energy excited state 3. Fluorescence • molecule returns to ground state by emitting a photon 4. Non-radiative relaxation • molecule returns to ground state but does not emit radiation pH sensitive fluorescent dyes • Fluorophores are aromatic or conjugated with delocalized electrons • pH sensitive fluorophores – emission differs at different pH values • Fluorescein and tetramethylrhodamine (pH insensitive) are used frequently in cellular applications – high absorbance and emission wavelength in the visible light range Structures of pH-sensitive Dyes Highlighted groups changed with pH carboxy SNARF-1 Orgeon Green Fluorescein pH Insensitive Dyes pH insensitive dyes, such as tetramethylrhodamine, are used as a control Tetramethylrhodamine Experimental Results Fluorescence spectra of individual lipobeads containing fluorescein at varying pH levels: (a )pH = 5, (b) pH = 6 (c) pH = 7, (d) pH = 8. Dye Delivery • Fluorescent dyes can be encapsulated by phospholipid bilayer vesicles (liposomes). – Water soluble while retaining same reactivity – Protect dye from quenchers • Liposome vesicle fuses with cellular lipid bilayer and delivers dye inside cell for reactions – Also used for drug or gene delivery Typical Liposome Liposome containing Fluorescein Cellular data • Mice macrophages were incubated with fluorescent pH-sensing lipobeads – Lipobead is a membrane on a polystyrene bead – Lipobeads filled with fluorescein and tetramethylrhodamine • Dyes allowed to interact endocytosed allowing dyes to interact with intracellular environment • Cells were analyzed with fluorescence-imaging microscopy – Exposure to detection light • Intracellular pH is determined from emission peaks Cellular Data Mice macrophages loaded with fluorescent lipobeads under bright field (left) and fluorescent imaging (right) under x40. Cellular Data pH change of a single liposome of fluorescent marker: • A sharp drop in fluorsecence is observed (t=9 sec) when the cell ingests the dye into a more acidic environment. • The more acidic environment causes fluorescein fluorescence to decrease. Advantages & Disadvantages • Advantages – No leaking like other methods (polymer matrix) – High chemical stability in solution – Protection of dye from quenching species • Disadvantges – Biocompatability/Cytotoxicity