* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download document
George S. Hammond wikipedia , lookup
Ring-closing metathesis wikipedia , lookup
Hydrogenation wikipedia , lookup
Asymmetric induction wikipedia , lookup
Stille reaction wikipedia , lookup
Strychnine total synthesis wikipedia , lookup
Tiffeneau–Demjanov rearrangement wikipedia , lookup
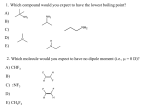
Organic Chemistry Reviews Chapter 7 Cindy Boulton November 2, 2009 Nomenclature of Alkynes Carbon – Carbon triple bond Ending –yne sp hybridized Linear and angle = 1800 Number the bond with the carbon that has the lower number Terminal Alkyne: a triple bond at a terminal carbon Has a acetylenic proton Acetylenic Proton: Proton at the end attached to a Carbon with a triple bond Easily pulled off with a pKa value = 25 Nomenclature of alkenes Carbon – Carbon double bond Ending –ene sp2 hybridized All atoms are coplanar and angle = 1200 Double bond cannot rotate Number the bond with the carbon that has the lower number Cis: same groups on SAME side Trans: same groups on OPPOSITE side Diasteromers: Same molecular formula, same connectivity, not mirror images E-Z Nomenclature If no 2 groups are the same, cannot use cis or trans Identify the highest priority (highest mass) group attached to each Carbon. (Z): SAME side Zame Zide (E): OPPOSITE side Vinyl and Allyl Groups Vinyl Group CH2 = CH – Allyl Group CH2 = CH – CH2 – Stability of Alkenes Alkyl groups provide electron density to stabilize the alkene Hydrogen does not provide electron density Electronics: more electron donors, more stable Sterics: more crowding, less stable The greater number of attached alkyl groups or the more highly substituted the carbon atoms of the double bond, the greater is the alkene’s stability Stability of Alkenes cont. Tetrasubstituted: 4 alkyl groups attached Trisubstiuted: 3 alkyl groups attached Disubstitued: 2 alkyl groups attached On same carbon (3o Carbon) Trans Cis Monosubstituted: 1 alkyl group attached Unsubstitued: no alkyl groups attached (In order of decreasing stability) Stability of Cycloalkenes and Cycloalkynes Angle Strain 8 is the magic number Cycloalkenes: Cyclopropene to Cycloheptene Cyclooctene Angle strain Must be in cis form (not stable in trans form) First stable cycloalkene Tans at double bond Cycloalkynes Cyclooctyne Can isolate at room temperature Unstable due to angle strain Wants to be linear (180o) but is 145o Synthesis of Alkenes Dehydrohalogenation reaction (E2) α Carbon: Carbon with Halide/Leaving Group attached to β Carbon: Carbon directly attached to α Carbon, has β Hydrogens attached E2 mechanism: Leaving group leaves, Nucleophile/Base attacks β Hydrogen, double bond forms between α and β carbons Transition step, no carbocation intermediate Two Products: Zaitsev: small bases lead to more stable/substituted alkenes due to electronics Hoffman: big, bulky bases lead to less stable/substitued alkenes due to sterics and crowding SN2 Product also forms Stereochemistry of E2 reaction Anti periplanar transition state β Hydrogen needs to be oppostie the leaving group Enantiomers will have the same E-Z nomenclature after dehyrdohalogenation reaction Diastereomes will have opposite E-Z nomenclature after dehydrohalogenation reaction Dehydration of Alcohols Hydroxyl group becomes protonated by an acid forming H-O-H+ to make a good “leaving group” E1 mechanism: Acid is a catalyze H-O-H leaves and to form Carbocation intermediate H-O-H acts as “nucleophile” attacking β Hydrogen forming alkene Two Products: Hoffman Product: less stable/substituted with bulky base Zaitsev Product: more stable/substituted with small base Dehydration of Alcohols cont. Alkyl and hydride migration A hydride (H-) or alkyl group will migrate from a β Carbon to the α Carbocation to form a more stable Carbocation Skeletal rearrangement Multiple products will be formed Debromination of vic-dibromides Gem: halides on same carbon (twins) Vic: halides on adjacent carbons Reacts with Zn/H2O or 2NaI to form alkenes Vic-dibromide must be in antiperi planar form I- acts as nucleophile pulling off one of the Br as the other Br leaves forming an alkene Debromination of vic-dibromides cont. Why 2 NaI? The second I pulls the I off the I-Br complex forming I-I With only 1 the reaction will stall Enanitomers have same product, same E/Z nomenclature Diastereomers have different products, different E/Z nomenclature A racemic mixture of vic-dibromide would have a single product Terminal Alkynes Terminal Alkynes have an acetylenic proton with a pKa = 25 Reacts with a strong base Forms an acetylide LDA NaNH2 Carbonanion Use acetylide as a nucleophile to attack alkyl halides to make alkynes bigger Hard to add 3o RX because the elimination product will be major Hydrogenation of Alkenes Use Pt as catalyst: Can use Ni, Pd, Rh or others Lowers the activation energy to speed up the reaction H are added to the same face of the alkene Stereochemistry: Syn-Addition Z -> RS and SR E -> RR and SS Forms a racemic mixture of enantiomers Regiochemistry: 1, 2 addition Carbons of double bond are side by side Hydrogenation of Alkynes Pt as catalyst: Forms an alkene but can not stop so continues to form alkane Lindlar’s Catalyst: H2/Pd/CaCO3 Stops as alkene Ca prevents alkene from being hydrogenated Stereochemistry: syn-addition Hydrogens added to same side Form Z or Cis alkene Hydrogenation of Alkynes cont. H2/Ni2B as Catalyst: Stops as alkene B prevents alkene from being hydrogenated Stereochemistry: syn-addition Hydrogens added to same side Form Z or Cis alkene 1) Li, C2H5NH2 2) NH4Cl Sterochemistry: anti-addition Hydrogens added to opposite side Form E or Trans alkene