* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download (1) and New York University (2)
Ring-closing metathesis wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Fischer–Tropsch process wikipedia , lookup
Kinetic resolution wikipedia , lookup
Cluster chemistry wikipedia , lookup
Stille reaction wikipedia , lookup
Baylis–Hillman reaction wikipedia , lookup
Metal carbonyl wikipedia , lookup
Strychnine total synthesis wikipedia , lookup
Hydroformylation wikipedia , lookup
Asymmetric induction wikipedia , lookup
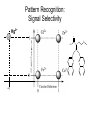
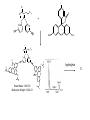
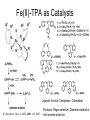
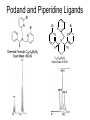
Molecular Recognition by PicolylamineBased Tripodal Ligands and Its Application in Sensing and Asymmetric Catalysis Amanda Mickley,1 Patrick Carney,1 Steven Lopez,2 Jennifer Lee,2 and Zhaohua Dai1,* Chemistry Departments of Pace University (1) and New York University (2) Objective • Improve the selectivity in the detection of Hg(II) through a stereochemical approach which systematically employs chiral podand, piperidine bearing ligands sulfur atoms; • Improve Hg(II) sensing sensitivity by using circularly polarized fluorescence excitation (CPE); • Systematically develope Fe(II) complexes of TPA-based chiral podands, piperidines and quinuclidines as green asymmetric catalysts for better product control and prediction in alkane hydroxylation by H2O2. Elucidate its mechanism • Elucidate mechanism to facilitate the better understanding of the interactions operating the above-mentioned molecular recognition events Chiroptical Fluorescence Sensors for Mercury 1st Genearation Sensor for Mercury Ratiometric Fluorescent Sensor Hg2+ Chiroptical Response HgII CuII CH3 Pattern Recognition: Signal Selectivity Hg2+ O S HO N N -1 N Next Generation Sensors for Hg2+ O HO O S HO N N HOOC N O O COOH 1 OH O O OH O O HOOC OH COOH N S HO N Zn 0 1 2 3 2+ Total Zn (M) 0 -5 H 200 220 240 260 280 300 320 340 (n m ) 2 + (R,R)-2 -5 -10 -15 -20 Zn -25 N H 5 lysozyme 0 2+ F (V) 10 O 5 18 16 14 12 10 8 6 4 2 0 F307 (V) d 15 F (V) O We intend to use these ligands to further develop circularly polarized fluorescence excitation (CPE), which is based on fluorescence-detected circular dichroism, which gives better contrast and eliminates many spectral interferences. (c) 2+ -30 200 220 240 260 280 300 320 340 (nm) I O S O O + N O N N AcO O OAc O S O N N N O O O O O hydrolysis 1. O O O O O O O Exact Mass: 1221.30 Molecular Weight: 1222.23 O O Alkane Hydroxylation R1 Chiral-TPA Fe(II) Complexes R1 * CH2 R2 CH H2O2 OH R2 • Converting saturated C-H bonds directly into alcohols • Important to synthetic organic chemistry, fuel industry and other industries using petrochemical feed stock • Helpful in modeling electron-transfer processes in biological systems, and producing new catalysts Fe(II)-TPA as Catalysts Ligands: Achiral; Complexes: Octahedral K. Chen and L. Que, Jr, JACS, 2001, 123, 6327 Products: Regio-selective, Diastereo-selective •Not enantio-selective Rigidification Enhances Enantiomeric and Regional Selectivity M. S. Chen, M. C. White. Science, 2007, 318, 783. Our Approach Make Chiral Ligands and use their Fe-(II) complexes as catalysts N N Systematically rigidify the chiral ligand to improve enantio- and regio- selectivity N N Put Br on ligands to increase reactivity according DFT calculation N N N H N H H Chiral podand N N H N H N N piperidine N N H H N H quinuclidine Podand and Piperidine Ligands Br Br N N N N H H C21H20Br2N4 Exact Mass: 486.01 Synthesis of Quinuclidines HC COOC2H5 CH + H2C H2C COOC2H5 COOC2H5 O 1) HCl, reflux C2H5OH + Na oxalyl chloride HC CH2 C OH COOC2H5 o 2) 190 C 3 Br N CH3NHOCH3 O HC CH2 C Cl O Br O Li CH3 HC CH2 C HC CH2 C N 3 OCH3 3 3 Br Br Br (+)DIP-Cl Br N HC CH2 CH 3 OH NH3 N MsCl HC CH2 CH N OMs Br N N H N H N o 3 50 C H 29 SSS-29 Br Br Br N N H N H N H C22H19Br3N4 m/e: 577.91 (100.0%), 579.91 (97.6%), 575.92 (34.3%), 581.91 (31.9%), SSS-29 Purity and Cu(II) Complex Acknowledgement • Prof. James Canary (NYU) • Prof. Demosthenese Athanasoupolos (Pace) • Kirill Grinberg (Midwood High School) Funding: ACS Petroleum Research Fund Research Corporation for Science Advancement Pace University Scholarly Research Fund Pace University Kenan Faculty Development Fund