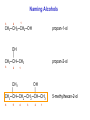* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download 02B naming alcohols and ethersFeb2013
Survey
Document related concepts
Transcript
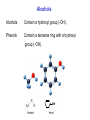
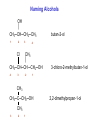
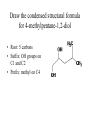
Alcohols Alcohols Contain a hydroxyl group (-OH). Phenols Contain a benzene ring with a hydroxyl group (-OH). OH OH + NaOH Phenol H2 O O- Na + + S od ium phenoxide (a w ater-soluble salt) Naming Alcohols CH4 methane CH3─OH methanol CH3─CH3 ethane CH3─CH2─OH ethanol Naming Alcohols Step 1- Identify the root Select the longest carbon chain that contains the -OH group. Name the parent alkane. Step 2 – Identify the suffix Number from the end nearest -OH group. The suffix of an alcohol always ends with –Ol. Indicate the position of each hydroxyl group. If more than one -OH group place a prefix (di, tri, tetra) at the beginning of the suffix Step 3- Identify the prefix Name and number any branches as you would for a hydrocarbon Step 4- Name the compound Combine the prefix, root, and suffix to name the compound. If the suffix begins with a vowel, drop the “e” on the end of the parent alkane. Name the following alcohol • • • • Parent alkane: heptane Suffix: -2,3-diol Prefix: 4-ethyl Name:4-methylheptane-2,3-diol Name the following alcohol • • • • Root: hexane Suffix: -1-ol Prefix:3-methyl Name: 3-methylhexan-1-ol Naming Alcohols 3 1 2 CH3─CH2─CH2─OH propan-1-ol OH │ CH3─CH─CH3 propan-2-ol 3 2 1 CH3 OH │ │ CH3─CH─CH2─CH2─CH─CH3 6 5 4 3 2 1 5-methylhexan-2-ol Naming Alcohols OH │ CH3─CH─CH2─CH3 1 2 3 butan-2-ol 4 CH3 │ CH3─CH─CH ─CH2─OH Cl 4 3 2 3-chloro-2-methylbutan-1-ol 1 CH3 CH3─C─CH2─OH CH3 3 2 1 2,2-dimethylpropan-1-ol Draw the condensed structural formula for 4-methylpentane-1,2-diol • Root: 5 carbons • Suffix: OH groups on C1 and C2 • Prefix: methyl on C4 Different types of Alcohols Primary (1º) Secondary (2º ) H │ CH3─C─OH │ H CH3 │ CH3─C─OH │ H 1C 2C attached to C-OH attached to C-OH Tertiary (3º) CH3 │ CH3─C─OH │ CH3 3C attached to C-OH Physical Properties of Alcohols 1. Alcohols are polar molecules (because of O-H and C-O). C-O: (3.5 – 2.5 = 1.0) O-H : (3.5 – 2.1 = 1.4) 2. Hydrogen bonding between alcohols molecules. 3. Have higher boiling points than Alkanes, Alkenes, and Alkynes. 4. Molecular weight ↑ : London dispersion forces ↑ : bp ↑ 5. More soluble in water than alkanes (Molecular weight ↑ : solubility ↓). polar nonpolar OH Oxidation of 1° Alcohols In the oxidation [O] of a primary alcohol 1, one H is removed from the –OH group and another H from the C bonded to the –OH. primary alcohol OH │ CH3─C─H │ H ethanol [O] K2Cr2O7 H2SO4 aldehyde O ║ CH3─C─H + H2O ethanal Oxidation of 2° Alcohols The oxidation of 2 alcohols is similar to 1°, except that a ketone is formed. [O] secondary alcohol OH │ CH3─C─CH3 │ H 2-propanol K2Cr2O7 H2SO4 ketone O ║ CH3─C─CH3 + H2O 2-propanone Oxidation of 3° Alcohols Tertiary 3 alcohols cannot be oxidized. [O] Tertiary alcohol OH │ CH3─C─CH3 no reaction K2Cr2O7 H2SO4 no product │ CH3 no H on the C-OH to oxidize 2-methylpropan-2-ol