* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Sugars
Survey
Document related concepts
Transcript
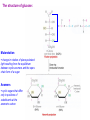
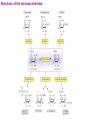
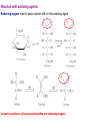
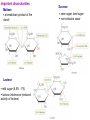
Sugars Alice Skoumalová Repetition: 1. Glucose, fructose and galactose (structure) 2. Optical isomerism - definition (D- and L-glucose) 3. Formation of a cyclic glucose (anomers) 4. Reducing sugars - definition 5. The most important disaccharides (composition) 6. The most important polysaccharides (structure) Important sugars in an organism: 1. Glucose 2. Glycogen 3. Glycoproteins 4. Glycolipids 5. Proteoglycans An introduction to carbohydrates: a large class of naturally occurring polyhydroxy aldehydes and ketones monosaccharides (simple sugars): from 3 to 7 carbons + one aldehyde or ketone functional group chiral compounds (having right- or left-handedness with two different mirror-image forms) because they contain carbons bonded to four different groups An aldose A ketose Monosaccharides, disaccharides, polysaccharides Modified saccharides The D and L families of sugars: Enantiomers - „mirror images“ (rotate polarized light in opposite directions → optical activity) Fischer projection: a mirror D sugar → the OH group on the chiral carbon farthest from the carbonyl group pointing to the right in a Fischer projection L sugar → the OH group on the chiral carbon farthest from the carbonyl group pointing to the right in a Fischer projection 2n of possible stereoisomers (half that many pairs of enantiomers) n - the number of chiral carbons (e.g. glucose - 16 stereoisomers) The structure of glucose: Mutarotation change in rotation of plane-polarized light resulting from the equilibrium between cyclic anomers and the openchain form of a sugar Anomers cyclic sugars that differ only in positions of substituents at the anomeric carbon Reactions of the monosaccharides: Reaction with oxidizing agents: Reducing sugars react in basic solution with a mild oxidizing agent In basic solutions, all monosaccharides are reducing sugars Reaction with alcohols (glycoside formation): Glycosidic bond → bond between the anomeric carbon of a monosaccharide and an -OR group Hydrolysis of a disaccharide (during digestion of carbohydrates) Important monosaccharides Aldoses: Pentoses in RNA and NADH in polysaccharides in the walls of plant cells Hexoses „blood“ sugar (energy) in cellulose and starch in glycogen (as a source of energy in an organism) in glycolipids and glycoproteins in lactose (milk), glycolipids and glycoproteins converted to glucose galactosemia Ketoses: D-Ribulose D-Fructose an intermediate in the pentose phoshate pathway in fruit juices and in honey in sucrose converted to glucose Deoxyaldoses: 2-deoxy-D-ribose in DNA Acetylated amino sugars: N-Acetyl-D-glucosamine N-Acetyl-D-galactosamine in glycoproteins Acidic sugars: D-Glucuronic acid N-Acetylneuraminic acid (sialic) in glycosaminoglycans in connective tissue conjugation of bile acids in glycoproteins Important disaccharides Maltose a breakdown product of the starch Lactose milk sugar (4,5% - 7%) lactose intolerance (reduced activity of lactase) Sucrose cane sugar, beet sugar non-reducing sugar Important polysaccharides Cellulose ß-D-Glucose, ß-1,4 link the fibrous substance that provides structure in plants humans cannot hydrolyze cellulose Starch α-D-Glucose source of energy in plants fully digestible - an essential part of the human diet (the grains wheat, potatoes, rice) 1. Amylose (20%, soluble in water) α-1,4 link 2. Amylopectin (80%, not water soluble) α-1,6 branches (every 25 units) Glycogen α-D-Glukose, α-1,4 and α-1,6 link source of energy in animals (liver, muscles) Other important sugars 1. Glycosaminoglycans Hyaluronic acid Chondroitin sulfate 25,000 disaccharide units in tendons and cartilage form very viscous mixture in connective tissue, synovial fluid, vitreous humour Glucuronic acid Glucuronic acid N-Acetylglucosamine N-Acetylgalactosamine sulfate Heparin contains sulfate groups (negative charges) anticoagulant - prevents the clotting of blood (binds to the clotting factors) 2. Proteoglycans aggregates of proteins and glycosaminoglycans in the extracellular matrix highly hydrated and resilient (cartilage) 3. Glycoproteins on the surfaces of cells (receptors, blood type) Forms: Blood type cell-surface carbohydrates Summary: 1. Epimers, enantiomers, anomers (examples) 2. The most important reaction of monosaccharides 3. The importance of glucose 4. Important disaccharides 5. Important polysaccharides 6. Glycosaminoglycans and proteoglycans - structure, function 7. Glycoproteins - structure, function