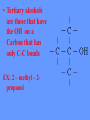* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Hydrocarbon Derivatives
Survey
Document related concepts
Transcript
• Hydrocarbon Derivatives And Functional Groups • Introduction – Hydrocarbon derivatives are formed when one or more hydrogen atoms is replaced by an element or a group of elements other than hydrogen. – Halogens (F2, Cl2, Br2, I2,) can all add to a hydrocarbon to form am alkyl halide. • • • • • When naming the halogen the –ine ending is replaced by –o Fluorine becomes fluoro Chlorine becomes chloro Bromine becomes bromo Iodine becomes iodo • Common examples of organic halides. – Alkenes can also add to each other in an addition reaction to form long chains of carbon compounds. • this is called polymerization – The atom or group of atoms that are added to the hydrocarbon are called functional groups. • Functional groups usually have multiple bonds or lone pairs of electrons that make them very reactive. • Alcohols – An alcohol has a hydrogen replaced by a hydroxyl (-OH) group. – The name of the hydrocarbon that was substituted determines the name of the alcohol. – The alcohol is named using the hydrocarbon name and adding the suffix –ol. • If methane is substituted with an OH group it becomes methanol • If a pentane group is substituted with an OH group it is pentanol. • For alcohols with more than two carbon atoms we need the number the chain so as to keep the alcohol group as low as possible. • Primary alcohols are those with the OH at the end of the Carbon chain. EX: ethanol ------------------------------------ 1-propanol -------------------------------------- • Secondary alcohols are those having the OH within the Carbon chain. EX: 2 - propanol ------------------------------ • Tertiary alcohols are those that have the OH on a Carbon that has only C-C bonds EX: 2 – methyl – 2propanol • Gasoline is a mixture of hydrocarbons (C8H18 for example) that contain no atoms of oxygen. Gasohol contains ethyl alcohol, C2H5OH, which does contain oxygen. • The addition of alcohol to gasoline, therefore, adds oxygen to the fuel. Since carbon monoxide forms when there is an insufficient supply of oxygen, the addition of alcohol to gasoline helps cut down on carbon monoxide emissions. If an alcohol contains two OH groups it is a diol (sometimes called a glycol). An alcohol with three OH groups is called a triol (sometimes called a glycerol). Addition Reactions • A substance is added to the double or triple bond of an alkene or alkyne. • The addition of water to an alkene is called a HYDRATION REACTION. To make alcohol add water to ethene H H C=C H H ethene + H2O water H OH H–C–C–H H H ethanol • The addition of HYDROGEN to an alkene is called a HYDROGENATION REACTION. H H C=C H H ethene H H + H2 H – C – C – H H H hydrogen ethane Ethers, Aldehydes, and Ketones Ethers An ether has a general formula R-O-R’. Use “ether” ending. EX: Diethyl ether C4H10O CH3CH2-O-CH2CH3 • The best-known ether is the anesthetic called diethyl ether. • Other ethers have a wide range of uses as solvents, refrigerants, artificial flavours, and drugs. • As a class of compounds, ethers are relatively unreactive chemically. • Ethers are isometric with the alcohols. • For example, diethyl ether (CH3CH2-OCH2CH3) is an isomer of butanol (CH3CH2CH2CH2-OH). • Both have the molecular formula C4H10O. Naming Ethers • Ethers are named in two ways: the common naming system, and the IUPAC naming system. • Common Names: The alkyl groups attached to the ether linkage are named in alphabetical order and are followed by the word ether. Symmetrical ethers are named by using the prefix di-. • IUPAC Names: The carbon(s) attached to the oxygen atom are named as branches by adding the – oxy suffix to the stem name. • Ex. CH3-CH2-CH2-CH2-O-CH2-CH3 • Common Name: butyl ethyl ether • IUPAC Name: ethoxybutane Aldehydes and Ketones • Two families of organic compounds, called aldehydes and ketones contain the carbonyl functional group (-C=O). • This group consists of a carbon atom double bonded to an oxygen atom. –An aldehyde has a carbonyl group (carbon double bonded to an oxygen) attached to a terminal carbon atom. Use “al” ending. • EX: Methanal CH2 O • H2C=O Uses of Aldehydes • The smaller aldehyde molecules have sharp, irritating odours. • The larger ones have flowery odours and are diluted to make perfumes. • Methanal is a starting material in the manufacture of some plastics. – A ketone has a carbonyl group attached to an internal carbon atom. Use “one” ending. • EX: Propanone C3H6O CH3 C=O CH3 • The carbonyl group (A) is present in both aldehydes and ketones, as shown in (B). (C) The simplest example of each, with the IUPAC name above and the common name below each formula. Uses of Ketones • The simplest ketone is propanone (common name = acetone). • It is an effective solvent found in many nail polish removers, plastic cements, resins and varnishes. • It is also sold as a cleaner. Because it is both volatile and flammable, it should be used only in well-ventilated areas. Organic Acids and Esters Organic acids are those acids that are derived from living organisms, usually from metabolism, but sometimes as a defense mechanism. Long chain organic acids are known as fatty acids. These are also called carboxylic acids as they contain the carboxyl functional group (COOH) One oxygen is double bonded to the carbon and the other is bonded to the carbon and to the hydrogen both with single • These red ants, like other ants, make the simplest of the organic acids, formic acid. • The sting of bees, ants, and some plants contains formic acid, along with some other irritating materials. Formic acid is HCOOH. –Esters are condensation products of carboxylic acids with the removal of water (also called a dehydration synthesis).