* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Alcohols
Survey
Document related concepts
Transcript
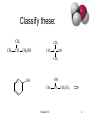
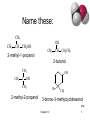
CHE 242 Unit V Structure and Reactions of Alcohols, Ethers and Epoxides; Basic Principles of NMR Spectroscopy CHAPTER TEN Terrence P. Sherlock Burlington County College 2004 Classify these: CH3 CH3 CH3 CH CH2OH CH3 C OH CH3 OH OH CH3 Chapter 10 CH CH2CH3 => 2 Name these: CH3 CH3 CH CH2OH 2-methyl-1-propanol OH CH3 CH CH2CH3 2-butanol CH3 CH3 OH C OH CH3 2-methyl-2-propanol Br CH3 3-bromo-3-methylcyclohexanol => Chapter 10 3 Naming Priority • • • • • • Acids Esters Aldehydes Ketones Alcohols Amines • • • • • Alkenes Alkynes Alkanes Ethers Halides => Chapter 10 4 Hydroxy Substituent • When -OH is part of a higher priority class of compound, it is named as hydroxy. • Example: OH CH2CH2CH2COOH also known as GHB 4-hydroxybutanoic acid => Chapter 10 5 Glycols • 1, 2 diols (vicinal diols) are called glycols. • Common names for glycols use the name of the alkene from which they were made. CH2CH2 CH2CH2CH3 OH OH OH OH 1,2-ethanediol 1,2-propanediol ethylene glycol propylene glycol Chapter 10 => 6 Naming Phenols • -OH group is assumed to be on carbon 1. • For common names of disubstituted phenols, use ortho- for 1,2; meta- for 1,3; and para- for 1,4. OH • Methyl phenols are cresols. OH H3C 4-methylphenol para-cresol Cl 3-chlorophenol meta-chlorophenol => Chapter 10 7 Physical Properties • Unusually high boiling points due to hydrogen bonding between molecules. • Small alcohols are miscible in water, but solubility decreases as the size of the alkyl group increases. => Chapter 10 8 Boiling Points => Chapter 10 9 Solubility in Water Solubility decreases as the size of the alkyl group increases. Chapter 10 => 10 Acidity of Alcohols • pKa range: 15.5-18.0 (water: 15.7) • Acidity decreases as alkyl group increases. • Halogens increase the acidity. • Phenol is 100 million times more acidic than cyclohexanol! => Chapter 10 11 Table of Ka Values CH3 OH => Chapter 10 12 Formation of Alkoxide Ions React methanol and ethanol with sodium metal (redox reaction). CH3CH2OH + Na CH3CH2O Na 1 + /2 H2 React less acidic alcohols with more reactive potassium. (CH3)3C OH + (CH3)3CO K K + 1/2 H2 => Chapter 10 13 Formation of Phenoxide Ion Phenol reacts with hydroxide ions to form phenoxide ions - no redox is necessary. O O H + OH + HOH pKa = 15.7 pKa = 10 => Chapter 10 14 Synthesis (Review) • Nucleophilic substitution of OH- on alkyl halide • Hydration of alkenes water in acid solution (not very effective) oxymercuration - demercuration hydroboration - oxidation => Chapter 10 15 Glycols (Review) • Syn hydroxylation of alkenes osmium tetroxide, hydrogen peroxide cold, dilute, basic potassium permanganate • Anti hydroxylation of alkenes peroxyacids, hydrolysis => Chapter 10 16 Organometallic Reagents • Carbon is bonded to a metal (Mg or Li). • Carbon is nucleophilic (partially negative). • It will attack a partially positive carbon. C - X C = O • A new carbon-carbon bond forms. => Chapter 10 17 Grignard Reagents • • • • Formula R-Mg-X (reacts like R:- +MgX) Stabilized by anhydrous ether Iodides most reactive May be formed from any halide primary secondary tertiary vinyl aryl => Chapter 10 18 Some Grignard Reagents Br + ether Mg Cl CH3CHCH2CH3 + Mg CH3C CH2 Br + Mg ether ether MgBr MgCl CH3CHCH2CH3 CH3C CH2 => MgBr Chapter 10 19 Organolithium Reagents • Formula R-Li (reacts like R:- +Li) • Can be produced from alkyl, vinyl, or aryl halides, just like Grignard reagents. • Ether not necessary, wide variety of solvents can be used. => Chapter 10 20 Reaction with Carbonyl • R:- attacks the partially positive carbon in the carbonyl. • The intermediate is an alkoxide ion. • Addition of water or dilute acid protonates the alkoxide to produce an alcohol. R C O R C O R C OH HOH Chapter 10 => 21 OH Synthesis of 1° Alcohols Grignard + formaldehyde yields a primary alcohol with one additional carbon. CH3 H3C C CH2 C H H CH3 H H MgBr C O CH3 CH CH2 H CH2 H MgBr H CH3 CH3 C O CH CH2 H CH2 HOH C O H H => Chapter 10 22 Synthesis of 2º Alcohols Grignard + aldehyde yields a secondary alcohol. CH3 H3C C CH2 C H H CH3 H3C H MgBr C O CH3 CH CH2 CH3 CH2 H MgBr H CH3 CH3 C O CH CH2 CH3 CH2 HOH C O H H => Chapter 10 23 Synthesis of 3º Alcohols Grignard + ketone yields a tertiary alcohol. CH3 H3C C CH2 C H H CH3 H3C H MgBr C O CH3 CH CH2 CH3 CH2 H3C MgBr CH3 CH3 CH3 C O CH CH2 CH3 CH2 HOH C O H CH3 => Chapter 10 24 How would you synthesize… OH CH2OH CH3CH2CHCH2CH2CH3 OH OH CH3 C CH3 CH2CH3 Chapter 10 => 25 Grignard Reactions with Acid Chlorides and Esters • Use two moles of Grignard reagent. • The product is a tertiary alcohol with two identical alkyl groups. • Reaction with one mole of Grignard reagent produces a ketone intermediate, which reacts with the second mole of Grignard reagent. => Chapter 10 26 Grignard + Acid Chloride (1) • Grignard attacks the carbonyl. • Chloride ion leaves. CH3 H3C R MgBr C O Cl CH3 R C O R C O MgBr Cl CH3 MgBr R C Cl + MgBrCl O Ketone intermediate Chapter 10 => 27 Grignard and Ester (1) • Grignard attacks the carbonyl. • Alkoxide ion leaves! ? ! CH3 H3C R MgBr C O R C O CH3O OCH3 CH3 R C O OCH3 MgBr CH3 MgBr R C + O MgBrOCH3 Ketone intermediate Chapter 10 => 28 Second step of reaction • Second mole of Grignard reacts with the ketone intermediate to form an alkoxide ion. • Alkoxide ion is protonated with dilute acid. CH3 CH3 R MgBr + R C R C O O MgBr R HOH CH3 R C OH R Chapter 10 => 29 How would you synthesize... Using an acid chloride or ester. OH CH3 CH3CH2CCH3 C CH3 OH OH CH3CH2CHCH2CH3 Chapter 10 => 30 Grignard Reagent + Ethylene Oxide • Epoxides are unusually reactive ethers. • Product is a 1º alcohol with 2 additional carbons. O O MgBr + CH2 MgBr CH2CH2 CH2 HOH O H CH2CH2 Chapter 10 => 31 Limitations of Grignard • No water or other acidic protons like O-H, N-H, S-H, or -C—C-H. Grignard reagent is destroyed, becomes an alkane. • No other electrophilic multiple bonds, like C=N, C—N, S=O, or N=O. => Chapter 10 32 Reduction of Carbonyl • Reduction of aldehyde yields 1º alcohol. • Reduction of ketone yields 2º alcohol. • Reagents: Sodium borohydride, NaBH4 Lithium aluminum hydride, LiAlH4 Raney nickel => Chapter 10 33 Sodium Borohydride • Hydride ion, H , attacks the carbonyl carbon, forming an alkoxide ion. • Then the alkoxide ion is protonated by dilute acid. • Only reacts with carbonyl of aldehyde or ketone, not with carbonyls of esters or carboxylic acids. O C H H C H H O + H H3O O H C H => Chapter 10 34 Lithium Aluminum Hydride • Stronger reducing agent than sodium borohydride, but dangerous to work with. • Converts esters and acids to 1º alcohols. O C OCH3 H LAH H3O+ Chapter 10 C O H H => 35 Comparison of Reducing Agents • LiAlH4 is stronger. • LiAlH4 reduces more stable compounds which are resistant to reduction. => Chapter 10 36 Catalytic Hydrogenation • Add H2 with Raney nickel catalyst. • Also reduces any C=C bonds. OH O OH H2, Raney Ni NaBH4 => Chapter 10 37 POWER POINT IMAGES FROM “ORGANIC CHEMISTRY, 5TH EDITION” L.G. WADE ALL MATERIALS USED WITH PERMISSION OF AUTHOR PRESENTATION ADAPTED FOR BURLINGTON COUNTY COLLEGE ORGANIC CHEMISTRY COURSE BY: ANNALICIA POEHLER STEFANIE LAYMAN CALY MARTIN Chapter 10 38