* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Lecture:Moisture
Thermometer wikipedia , lookup
Adiabatic process wikipedia , lookup
Automated airport weather station wikipedia , lookup
Tectonic–climatic interaction wikipedia , lookup
Evaporative cooler wikipedia , lookup
Satellite temperature measurements wikipedia , lookup
Hyperthermia wikipedia , lookup
Indoor air quality wikipedia , lookup
Atmospheric circulation wikipedia , lookup
Cold-air damming wikipedia , lookup
Atmosphere of Earth wikipedia , lookup
Surface weather analysis wikipedia , lookup
Water vapor wikipedia , lookup
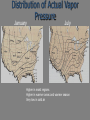
Atmospheric Moisture CH 5. pp. 122-152 Hydrologic Cycle Water is an Odd Molecule Boiling point is much higher than one would expect from molecular weight. Substance Oxygen Nitrogen Water Carbon Dioxide Molecular weight 32 28 18 44 Boiling Temp -183 oC -196 oC 100 oC -79 oC Water is a Polar Molecule Net positive charge on one side and a negative side on the other. Polar Molecule Electrical negative will attract positive charge. The electrical attraction of the polar molecules is quite strong. The attraction makes water molecules join together, raising the temperature of the boiling and freezing point. Polar attraction also makes water a good liquid solvent. Other molecules bind to water in solution. Water one of the very few substances that is densest in its liquid state Implications in the atmosphere? Ice Crystal 105 degree separation of hydrogen causes formation of a six sided crystal. Water Vapor As the number of water vapor molecules in the air increase in number, they will have more chance of being close to another molecule and being attracted to each other. After there are a certain number of molecules in the air, any more molecules will cause the molecules to clump together forming a liquid. This point is the saturation point. Saturation Point Saturation point dependent upon: Temperature (the higher the temperature the more the molecules can escape from each other’s influence) Pressure (the higher the pressure, the higher the number of molecules present) Atmospheric Moisture Methods of achieving saturation 1. Adding water vapor to the air 2. Mixing cold air with warm, moist air 3. Lowering the temperature to the dew point Let’s talk about #3 Air Temperature can change by : Diabatic & Adiabatic Processes Atmospheric Moisture Diabatic Processes Processes that involve the removal/input of heat Increase Heat; Increase Temp.; Increase volume Adiabatic Processes Processes that do not involve the removal/input of heat Expansion of air; Increase volume; Decreases Temp. Evaporation/Condensation Imagine a container of water covered with a lid Some molecules go from liquid to gas (evaporation), and some go from gas to liquid (condensation). The air above the liqud surface is at saturation when the number of molecules escaping equals the number of molecules reentering the water. Saturation Saturation occurs when evaporation=condensation (the air can’t hold any more water vapor) Increasing the temperature of the air increases the amount of water vapor it can hold (it takes more water vapor to reach saturation point) Moisture Measurements There are a number of measurements we can use to specify the amount of moisture (also referred to as humidity) in the air: absolute humidity specific humidity vapor pressure saturation vapor pressure relative humidity mixing ratio saturation mixing ratio wet-bulb temperature dew-point temperature Absolute Humidity Absolute Humidity = mass of water vapor/volume of air So, absolute humidity is like a water vapor density, commonly express in grams/m3 Abs humidity is not a useful measurement for humidity--because it changes with volume and temperature changes that occur in the atmosphere. Why? Expansion/Compression What happens to the volume of a rising or sinking air parcel? Consider a parcel of air at 1000mb The parcel exerts 1000 mb of outward pressure to counteract the atmospheric pressure acting on the parcel If no energy is added or taken away from the parcel, then the force of the molecules bumping into the side of the parcel will be constant Expansion Now, we raise the parcel to 500 mb The outside pressure on the parcel decreases, so the volume of the parcel increases as the parcel expands Specific Humidity Specific humidity = mass of water vapor/total mass of air For example, in a parcel, the mass of water vapor is 1g The total mass of the parcel (N2, O2, Ar, H2O, other trace gasses) is 1 kg Mass H20/Total mass of the parcel of air (including the water vapor) Specific Humidity is 1g/kg Specific humidity is not effected by expansion and compression changes in the air parcel Much more useful in meteorology Latitudinal Distribution of Specific Humidity Figure 5.9 High at the equator Low at the poles Absolute & Specific Humidity Figure 5.7 Specific humidity is concerned with the mass of vapor to mass of air, and is not affected by changes in parcel volume. For a given mass of water vapor in an air parcel, the absolute humidity changes as the parcel volume changes (lifts or descends). Figure 5.8 Mixing Ratio Mixing ratio = mass of water vapor/mass of dry air For example: In a parcel, the mass of water vapor is 1g; and the mass of the dry air in the parcel is 1.0 kg The mixing ratio is 1g/kg Vapor Pressure Remember from Chap 1 that pressure is the force of collisions of molecules against a surface The total pressure is the sum of the pressures of the different molecules If the total pressure in this parcel was 1000 mb-Nitrogen would contribute 780 mb, Oxygen 210 mb, and Water vapor 10 mb. Actual Vapor Pressure (Dalton’s Law) The total pressure of the air parcel is due to the sum of partial pressures of each of the gasses comprising the parcel The pressure due to water vapor is called the actual vapor pressure Distribution of Actual Vapor Pressure January July Higher in moist regions Higher in warmer areas and warmer season Very low in cold air Saturation Vapor Pressure Actual vapor pressure tells us the total water vapor content of the air.. Saturation vapor pressure indicates how much water vapor pressure is present when the air is saturated Dependent on air temperature It takes less moisture to saturate a cold parcel Warm air can hold much more water vapor than cold air Relative Humidity How much water vapor is there divided by how much it can hold (X 100)=RH Relative Humidity can be calculated by: Basically: Content/capacity Actual vapor pressure/saturation vapor pressure Actual mixing ratio/saturation mixing ratio We can change RH in two ways: Change the amount of vapor in the air OR Change the air temperature Relative Humidity is affected by both temperature and pressure Relative humidity is high at equator and poles In most places, vapor content changes only slightly over a day, yet RH varies widely each day This is due to daily change in air temperature. Lowest temperatures of early morning result in highest RH values, warm afternoon result in lowest RH values Relative and Specific Humidity Relative humidity (RH) as an indicator of saturation reveals that desert air is far from saturated, and that cold polar air nears saturation. Graphs of RH contrast with specific humidity in the deserts and poles. Specific Humidity Relative Humidity Figure 5.14 Dew Point Temp (TDP) The temperature that the air must be cooled to (assuming no changes in water vapor or pressure) in order for saturation to occur If the dew point is < 32oF, it’s called Frost Point Important measurement to predict formation of dew, frost, fog and minimum air temperatures…as well as a good indicator of severe weather, cumulus cloud ceiling heights High TDP is a good indicator of the air’s actual vapor content. Seasonal Dew Point Maps Figure 5.12A Figure 5.12B January, July Average Dew Point Temperatures -High Dew Points=plentiful moisture -Low Dew Points=very dry air Dew Point Reality Can you feel dew point? On a typical summer day the following is true: Dew Point(F) Perception 75+..........................Extremely uncomfortable 70-74........................Very humid, quite uncomfortable 65-69........................A bit uncomfortable for most people 60-64........................Ok for most, but everyone begins to feel the humidity 55-59........................Comfortable 50-54........................Very comfortable <=49........................”Feels like the west”, very pleasant, feels a bit dry to some Wet Bulb Temperature The lowest temperature that can be reached by evaporating water into the air Note: the wet bulb temperature will always be less than or equal to the temperature If you are a runner: A good measure of how cool the skin can become by sweating T = 90°F, RH = 90%...High wet-bulb temperature. T = 90°F, RH = 10%...Low wet-bulb temperature. You feel more comfortable when wet-bulb temperature is low Wet-bulb temperature is related to the heat index Measuring Moisture Moisture Measurements Hair Hygrometer Human hair lengthens with increasing RH. (why you have a “bad hair day”) Levers amplify the change in length. Measures Relative Humidity directly. Relatively cheap instrument. Moisture Measurements Sling Psychrometer Two thermometers; one wet, one dry. Move through the air (either fan or swing around). Wet thermometer measures wet bulb temperature Moisture measurements Dew Point Cool mirror until dew (or frost) forms on mirror. Used by NWS at all automated surface observing stations. Moisture Measurements Electrical Hygrometer Uses a chemical film that absorbs moisture which changes the electrical resistance. Used in radiosonde measurements.