* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Slide 1
Persistent carbene wikipedia , lookup
Oxidation state wikipedia , lookup
Ring-closing metathesis wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Metal carbonyl wikipedia , lookup
Spin crossover wikipedia , lookup
Coordination complex wikipedia , lookup
Stability constants of complexes wikipedia , lookup
Metalloprotein wikipedia , lookup
Stille reaction wikipedia , lookup
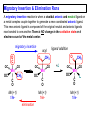
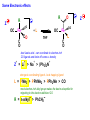
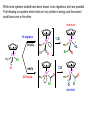
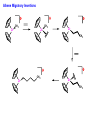
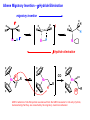
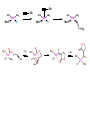
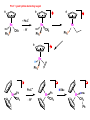
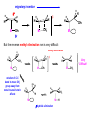
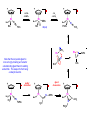
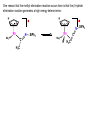
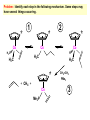
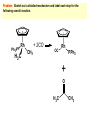
Migratory Insertion & Elimination Rxns A migratory insertion reaction is when a cisoidal anionic and neutral ligand on a metal complex couple together to generate a new coordinated anionic ligand. This new anionic ligand is composed of the original neutral and anionic ligands now bonded to one another.There is NO change in the oxidation state or d electron-count of the metal center. migratory insertion O O C OC Mn OC C O OC CO Mn(+1) 18eelimination O CH3 Mn CO +L OC CH3 Mn OC OC CH3 ligand addition acyl C O C O Mn(+1) 16e- Mn(+1) 18e- CO L General Features of Migratory Insertions: 1) No change in formal oxidation state (exception: alkylidenes) 2) The two groups that react must be cisoidal to one another 3) A vacant coordination site is generated by the migratory insertion. Therefore, a vacant site is required for the back elimination reaction (e.g., b-hydride elimination). A trapping ligand is often needed to coordinate to the empty site formed from a migratory insertion in order to stop the back elimination reaction. 4) Migratory insertions are usually favored on more electron-deficient metal centers. The following are common anionic and neutral ligands that can do migratory insertion reactions with one another: Anionic: H-, R- (alkyl), Ar- (aryl), acyl-, O2- (oxo) Neutral: CO, alkenes, alkynes, carbenes CO and alkyl migratory insertions (as shown on previous slide) are extremely important and are often generically referred to as carbonylation reactions. Hydride and CO migratory insertions to produce formyl groups are not common due to the thermodynamic instability of the formyl-metal interaction. Some Electronic effects R R Z OC CO Fe C O +L CO OC O Fe CO L THF C O best Lewis acid - can coordinate to electron-rich CO ligands and drain off some e- density + Z + = Li > Na + + > (Ph3)2N strongest coordinating ligand - best trapping ligand L = PMe3 > PPhMe2 > PPh2Me > CO most electron-rich alkyl group makes the best nucleophile for migrating to the electron-deficient CO R = n-alkyl- > PhCH2- Z Migration vs. Insertion Migration O O C OC CO Mn OC CH3 C O Mn(+1) 18e- OC CH3 Mn OC C O Mn(+1) 16e- CO a MIGRATION rxn involves the anionic ligand doing a nucleophillic-like attack on the neutral ligand. This involves the anionic ligand moving to the site where the neutral ligand is coordinated. An empty coordination site is left behind. Insertion O C OC Mn OC C O Mn(+1) 18e- CO CH3 an INSERTION rxn involves the neutral ligand moving over to OC CO Mn O where the anionic ligand is coordinated and "inserting" into OC the anionic ligand-metal bond to C CH3 generate the new anionic O ligand. An empty coordination site is left behind from where Mn(+1) the neutral ligand originally was 16elocated. While most systems studied have been shown to do migrations, both are possible. The following is a system where both are very similar in energy and the solvent used favors one or the other. inversion Et migrates CH3NO2 Ph3P Fe* Ph3P Ph3P Et Fe* *CO Fe* Et O C*O O Et C O HMPA CO inserts Fe* Ph3P Et *CO O Fe* Ph3P *C O Et retention O Alkene Migratory Insertions Zr CH 3 Zr CH3 Zr CH 3 Zr CH3 Zr CH3 Alkene Migratory Insertion – b-Hydride Elimination migratory insertion H H H H M H H H ‡ H H H H H H M H H M b-hydride elimination * * Nb H * CO R Nb CO Nb R H * * * H R N MR ir r adiat ion of t he Nb-hy dr ide r esonance af f ects the NMR r esonance f or t he alk yl hydr ide, demonst rating t hat t hey ar e connect ed by t he migr at or y inser tion mechanism Problem: Why don’t either of the complexes shown below do alkene-hydride migratory insertions at room temperature? H Cl Ir Ph3P PPh3 CO Et3P Pt H PEt3 Problem: Sketch out and label the two mechanistic steps (in the correct order) that are occurring for the following reaction. Ru H C O Ru + PPh3 Ph3P C O CH3 RCN Rh Me3P CH3 PMe3 RCN Rh H Me3P PMe3 RCN Rh H PMe3 Me3P CH3 O O O Ph3P -PPh3 O Pd Cl O Ph3P Ph3P Pd Pd PPh3 Me Ph3P O Cl Cl O Me O +PPh3 Pd Cl PPh3 Agostic C-H to Metal Interactions – “Frozen Migratory Insertion” * Co R3P * * + H+ Co Co H R3P H H H R3P One of the C-H bonds of the methyl group is within bonding distance to the Co center. This is called an Agostic C-H bond interaction. Because the C-H bond is sharing some of its s-bond electron density with the metal, the C-H bond is weakened. This produces some relatively clear-cut spectroscopic characteristics: 1) nC-H infrared stretching frequency is lowered to the mid-2500 cm-1 region from a normal value of 2900-3000 cm-1 2) the JC-H coupling constant in the 13C NMR is lowered to around 70-90 Hz from a normal value of 150 Hz. 3) the 1H chemical shift of the agostic proton is in the -10 to -15 ppm region, much like a metal-hydride resonance. Carbene-Alkylidene Migratory Insertions X M CH2 + L L X M CH2 X = H-, R-, OR-, halideNormally a migratory insertion refers to a neutral ligand reacting with an anionic ligand to produce a new anionic ligand. But if we electron-count the carbene as a dianionic ligand (alkylidene), we are reacting a monoanionic ligand (X) with a dianionic ligand (alkylidene) to make a new monoanionic ligand. This changes the oxidation state of the metal center and is now formally a reductive coupling reaction. In the case of X = H-, the reverse reaction is called an a-hydride abstraction or elimination. Ph3C+ = good hydride abstracting reagent * * * + Ph3C+ Re H3C CH3 Ph3P Re - -H H3C Re CH2 Ph3P Ph3P * Re H Ph3P W Ph CH3 Ph3C W -H Ph CH2 NCMe W NCMe CH2 Ph Eliminations H H H b-hydride elimination H M M H H H M M R R O M a-hydride elimination R M CO carbonyl elimination or decarbonylation R The key points are: 1) No change in formal oxidation state (exception: alkylidenes) 2) You must have an empty orbital that is cisoidal to the group that you are doing an elimination reaction on. Alternatively, a cisoidal labile ligand that can easily dissociate to open up an empty orbital. migratory insertion H H H M H H H CH3 - ‡ H H H M H H H CH3 CH3 M But the reverse methyl elimination rxn is very difficult: H H H methyl elimination H H H CH3 M M rotation of C-C bond to move CH3 group away from metal to avoid steric effects H H CH3 H H H H ‡ H H H H CH3 M H H CH3 H M M b-hydride elimination H CH3 Very Difficult! One unusual example of what is believed to be a methyl elimination reaction is involved in the following transformation (Bergman, JACS, 2002, 124, 4192-4193): * * Rh Me3P H3C N + HSiPh3 C CH3 Rh Me3P H3C C + CH4 N SiPh3 * * - CH4 + HSiPh3 + NCCH3 Rh Me3P N H3C * - NCCH3 Me3P C Rh Me3P H H 3C CH3 Rh SiPh3 Rh(+5) Rh methyl elimination H3C ligand dissociation Rh C N Me3P SiPh3 N C H3C SiPh3 CH3 N C C * C Rh CH3 H3C Rh Me3P SiPh3 N Note that the isocyanide ligand is more strongly donating and a better -backbonding ligand than the starting acetonitrile. This keeps this from being a catalytic reaction. * N Ph3Si * Rh Me3P C N Ph3Si CH3 SiPh3 One reason that the methyl elimination reaction occurs here is that the b-hydride elimination reaction generates a high energy ketene-imine: * * N SiPh3 Rh Me3P N C H3C SiPh3 Rh Me3P H H2C C Problem: Identify each step in the following mechanism. Some steps may have several things occurring. * 1 * Co 2 * Co H3C Co H H3C H 3C H 3C * + CH4 + Co Me3P CH2=CH2 PMe3 3 Problem: Sketch out a detailed mechanism and label each step for the following overall reaction. Ph3P + 2CO Rh H3 C CH3 OC Rh PPh3 + O H3C CH3