* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Basilar artery aneurysm with autonomic features: an interesting
Feature detection (nervous system) wikipedia , lookup
Optogenetics wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Neuroesthetics wikipedia , lookup
Neuroanatomy wikipedia , lookup
Neuroregeneration wikipedia , lookup
Sports-related traumatic brain injury wikipedia , lookup
Clinical neurochemistry wikipedia , lookup
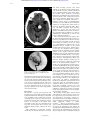
Downloaded from http://jnnp.bmj.com/ on October 13, 2016 - Published by group.bmj.com J Neurol Neurosurg Psychiatry 2001;71:805–808 805 SHORT REPORT Basilar artery aneurysm with autonomic features: an interesting pathophysiological problem N J GiYn, P J Goadsby Abstract Unruptured cerebral aneurysms often present with neuro-ophthalmological symptoms but ocular autonomic involvement from an aneurysm of the posterior circulation has not previously been reported. A patient is described with a basilar artery aneurysm presenting with headache and unilateral autonomic symptoms. After angiographic coiling of the aneurysm there was a near complete resolution of these features. The relevant anatomy and proposed mechanism of autonomic involvement of what may be considered—from a pathophysiological perspective as a secondary trigeminalautonomic cephalgia— is discussed (J Neurol Neurosurg Psychiatry 2001;71:805–808) Keywords: trigeminal autonomic cephalgia; cluster headache; trigeminovascular system Although the most common presentation of a cerebral aneurysm is with subarachnoid haemorrhage, unruptured cerebral aneurysms may present with headache or other symptoms, in particular neuro-ophthalmological features. These usually large, symptomatic aneurysms are important to detect as, unlike small (<10 mm) aneurysms, which have an annual rupture rate of 0.05%, those with a diameter greater than 25 mm have an annual rupture rate of 6%.1 2 The neuro-ophthalmological features depend on the size and location of the aneurysm relative to the visual pathways and other cranial nerves.3 However, autonomic features from a basilar artery aneurysm have not previously been described and have some interesting physiological implications for the trigeminal-autonomic syndromes. Headache Group, Institute of Neurology, Queen Square, London WC1N 3BG, UK N J GiYn P J Goadsby Correspondence to: Professor P J Goadsby [email protected] Received 14 November 2000 and in final form 18 June 2001 Accepted 6 August 2001 Case report A 61 year old woman presented with a 30 month history of a left sided headache. The headache started suddenly, was localised above the left eye and radiated occipitally, occurring for 3 to 14 days each month but with a residual background pain. For the year before presentation the pain had become daily, associated with left nasal stuYness, tearing and reddening of the eye, and drooping of the eyelid. She had noticed blurring of vision in the left eye for 4 months. Compound analgesics, ergotamine www.jnnp.com with caVeine, and 50 mg amitriptyline at night had not relieved the pain. She had a medical history of hypertension controlled on enalapril. Her mother had had headaches but the patient had no personal history of headaches. On examination there was a left sided ptosis, conjunctival injection, and lacrimation (fig 1). Direct and consensual pupillary responses to light and the accommodation reflex were normal. Pupillography was not performed. External ocular movements were normal with no diplopia. Fundoscopy was normal. She had no other neurological signs apart from a mild, long standing “no-no” head tremor. Her blood pressure was 120/80. Brain CT without contrast showed a large basilar artery aneurysm (fig 2) the location of which was confirmed on CT angiography as lying between the left superior cerebellar artery and the left posterior cerebral artery (fig 3). The aneurysm was successfully occluded with angiographic coil embolisation. By day 5 after embolisation the lacrimation, nasal stuYness, and conjunctival injection had resolved, the Figure 1 Patient before embolisation showing left sided ptosis (with permission). Downloaded from http://jnnp.bmj.com/ on October 13, 2016 - Published by group.bmj.com 806 GiYn, Goadsby Figure 2 CT without contrast showing the large aneurysm of the basilar artery. Figure 3 Pre-embolisation CT angiogram showing aneurysm lying between superior cerebellar artery and posterior cerebral artery. blurred vision and headache improved but ptosis persisted. Three months after embolisation she had only infrequent mild daily headache and the ptosis was less than on initial presentation. A repeat angiogram showed some compaction of the coils but an increase in the size of the neck compared with the previous angiogram. It is planned to repeat the angiogram at intervals in the future. Discussion We describe a patient who presented with headache in association with cranial autonomic symptoms manifested clinically by nasal stuYness, lacrimation and conjunctival injection (parasympathetic activation), and ptosis (sympathetic inactivation). We consider these symptoms to be secondary to the basilar artery aneurysm because there are anatomical pathways that would explain these features, and symptoms improved after coiling of the aneurysm. This combination of autonomic features, which may also include rhinorrhoea, forehead www.jnnp.com and facial sweating, meiosis, and eyelid oedema, are prominent in some primary headache disorders,4 the pain of which is mediated via the trigeminovascular system. These disorders include cluster headache, paroxysmal hemicrania and SUNCT (short lasting neuralgiform headache with conjunctival injection and tearing) syndrome, which may be collectively described as trigeminal autonomic cephalgias.5 A lesser degree of parasympathetic activation is not uncommonly seen in migraine and may be accompanied by cranial release of vasoactive intestinal polypeptide (VIP).6 However, autonomic activation from structures supplied by C1/C2 has only rarely been described in humans and presents an interesting pathophysiological challenge in terms of explaining the mechanisms at work in this patient’s clinical presentation. The sensory innervation of the pain producing intracranial vascular structures of the forebrain is by trigeminal nerve aVerents whereas those below the tentorium cerebelli are innervated mainly by branches of the C2 dorsal root.7 Trigeminal nerve aVerents relay via the trigeminal nucleus caudalis in the caudal medulla and in neurons of the dorsal horns at C1 and C2,8 the trigeminocervical complex. EVerents from the trigeminocervical complex synapse in the superior salivatory nucleus. Stimulation of these pain producing structures in experimental animals leads to c-fos expression, activation of neurons, in the superior salivatory nucleus.9 Parasympathetic fibres pass from the superior salivatory nucleus via the greater petrosal nerve component of the facial nerve to the sphenopalatine ganglion10 11 to mediate the parasympathetic features associated with trigeminally mediated pain. EVerent nitric oxide and VIP containing fibres from the sphenopalatine ganglion also produce vasodilatation of both cerebral and extracerebral arteries, including the meningeal arteries that are served by trigeminal aVerents. These connections complete a positive feedback loop for the exacerbation of vasodilatation and pain in primary headache disorders—the trigeminovascular reflex.12 In addition, parasympathetic mediated vasodilatation of the internal carotid artery may injure the surrounding plexus of sympathetic fibres en route to the eye and forehead. Thus trigeminal activation can cause simultaneous parasympathetic activation and sympathetic inactivation, probably accounting for the autonomic features seen in the trigeminal autonomic cephalgias. How might cervical inputs entrain cranial parasympathetic neurons? Indeed, there have been previous reports of structures with a cervical innervation—for instance, lesions produced from the lateral medullary syndrome, to trigger headache syndromes with cranial autonomic symptoms similar to cluster headache.13–15 This can be explained by convergence of aVerent trigeminal and C2 aVerents at the level of the trigeminocervical complex. In experimental animals stimulation of structures innervated by the trigeminal nerve, such as the superior sagittal sinus8 and middle meningeal Downloaded from http://jnnp.bmj.com/ on October 13, 2016 - Published by group.bmj.com 807 Basilar artery aneurysm with autonomic features artery,16 activates neurons in the trigeminocervical complex. Similarly, stimulation of the greater occipital nerve, a branch of C2, leads to activation of neurons in the very same regions.17 It has also been shown that stimulation of the greater occipital nerve in rats leads to ipsilateral conjunctival injection, tearing, and ptosis in one third of animals.18 Thus an anatomical pathway exists whereby C2 activation may trigger eVerent neurons from the trigeminocervical complex, including those to the superior salivatory nucleus that mediate parasympathetic activity. Neuro-ophthalmological symptoms from intracranial aneurysms are many and varied, depending on anatomical site and size and include visual loss, diplopia from III or VI nerve palsies, Horner’s syndrome, and symptoms from midbrain or pontine involvement. Visual loss may be caused by compressive injury to the optic nerve or chiasm from aneurysms of the anterior communicating, or the supraclinoid carotid artery. Diplopia is most commonly caused by a third nerve palsy from a posterior communicating artery aneurysm, but can also be caused by third nerve compression from cavernous, carotid, or basilar artery aneurysms or, more rarely, by sixth nerve compression of a vertebral artery aneurysm. Although we report sympathetic inactivation from a basilar aneurysm, Horner’s syndrome is usually caused by aneurysms of the carotid cavernous artery compressing the sympathetic nerve on its course through the cavernous sinus. Rarely, giant basilar bifurcation aneurysms or large mid-basilar aneurysms may compress the midbrain and pons causing neuroophthalmological signs including sixth nerve palsies, horizontal gaze paresis, skew deviation, internuclear ophthalmoplegia, lid retraction, and nystagmus typically in association with long tract brain stem signs such as hemiparesis or alterations in consciousness. Symptoms may also accrue from thrombus in a midbasilar aneurysm obstructing flow resulting in local pontine ischaemia. Thromboembolic disease may also result in widespread posterior circulation ischaemia, the top of the basilar syndrome, causing abnormalities of convergence and vertical gaze, skew deviation, visual field defects, visual hallucinations, amnesia, and delirium.3 The patient we describe had both head pain and features of parasympathetic activation and sympathetic impairment that we consider to be secondary to the aneurysm. We consider that her ptosis was secondary to sympathetic dysfunction rather than from third nerve compression because of the lack of diplopia and pupillary dilatation that would be typical of third nerve palsy. She did not show meiosis that would be typical of sympathetic dysfunction but this would be in keeping with primary trigeminal autonomic cephalgias that do not always have a full house of local autonomic features: lacrimation is the commonest feature reported in cluster headache (80%) followed by conjunctival injection (64%) with meiosis being apparent in less than 10%.19 However, we cannot rule out the possibility that there may have been subtle pupillary changes that may be www.jnnp.com missed without pharmacological pupillary testing. It is diYcult to know the exact cause of her blurring of vision, but it is likely to be due to a combination of lacrimation and subclinical pupillary change. The aneurysm was adjacent to the midbrain but we do not attribute her neuro-ophthalmological symptoms to compression of the brain stem or ischaemia because she did not have involvement of other cranial nerves or long tracts that would be expected to be aVected in a midbrain lesion. Similarly, ischaemic changes were not apparent on the patient’s MRI. We also consider that her headache was secondary to the aneurysm. Although the basilar artery only has sensory innervation from C2, the head pain that she described occurred in the distribution of the first division of the ipsilateral trigeminal nerve (supraorbital) as well as that supplied by C2 (occipital), again emphasising the cross talk between the trigeminal and cervical sensory systems in the trigeminocervical complex. Basilar artery aneurysms represent only 3%-5% of all intracranial aneurysms but are the most common aneurysms in the posterior fossa. Treatment of basilar aneurysms by angiographic embolisation with electrolytically detachable coils achieves a high success rate in suitable patients.20 However, periodic angiographic follow up after coil embolisation is recommended to identify aneurysm recurrence and those patients with a high risk of late rebleeding.21 The patient reported here had a basilar artery aneurysm causing headache combined with symptoms indicating autonomic dysfunction, features usually associated with stimulation of trigeminally innervated structures. This may be considered a secondary trigeminal autonomic cephalgia. Basilar aneurysms have not previously been reported to be associated with such features, but neuronal connections exist within the caudal brain stem that could mediate such an eVect. We cannot pretend to know, given the probably ubiquitous nature of the trigeminal-autonomic connections, why this particular lesion caused their activation when similar lesions do not necessarily cause this clinical picture. The patient’s presentation, however, provides a useful human confirmation of experimental animal studies that illustrate the physiology of the trigeminalautonomic reflex. The work reported has been supported by the Migraine Trust and the Wellcome Trust. NJG is supported by GlaxoSmithKline. PJG is a Wellcome Trust senior research fellow. 1 Wiebers D, Whisnant J, Forbes G, et al. Unruptured intracranial aneurysms - Risk of rupture and risks of surgical intervention. N Engl J Med 1998;339:1725–33. 2 Wardlaw JM, White PM. The detection and management of unruptured intracranial aneurysms. Brain 2000;123:205–21. 3 Kasner SE, Liu GT, Galetta SL. Neuro-ophthalmologic aspects of aneurysms. Neuroimaging Clin N Am 1997;7: 679–92. 4 Headache Classification Committee of the International Headache Society. Classification and diagnostic criteria for headache disorders, cranial neuralgias and facial pain. Cephalalgia 1988;8:1–93. 5 Goadsby PJ, Lipton RB. A review of paroxysmal hemicranias, SUNCT syndrome and other short lasting headaches with autonomic feature, including new cases. Brain 1997;120:193–209. 6 Goadsby PJ, Edvinsson L, Ekman R. Vasoactive peptide release in the extracerebral circulation of humans during migraine headache. Ann Neurol 1990;28:183–7. Downloaded from http://jnnp.bmj.com/ on October 13, 2016 - Published by group.bmj.com 808 GiYn, Goadsby 7 WolV HG. Headache and other head pain. New York: Oxford University Press, 1963. 8 Goadsby PJ, Hoskin KL. The distribution of trigeminovascular aVerents in the non-human primate brain Macaca nemestrina: A c-fos immunocytochemical study. J Anat 1997;190:367–75. 9 Knight YE, Goadsby PJ. Brainstem nuclei expressing c-fos after superior sagittal sinus stimulation: comparison with control and periaqueductal grey stimulation. Cephalalgia 2000;20:283. 10 Zhu BS, Gibbins IL, Blessing WW. Preganglionic parasympathetic neurons projecting to the sphenopalatine ganglion contain nitric oxide synthase in the rabbit. Brain Res 1997; 769:168–72. 11 Nakai M, Tamaki K, Ogata J, et al. Parasympathetic cerebrovasodilator center of the facial-nerve. Circ Res 1993;72:470–5. 12 May A, Goadsby PJ. The trigeminovascular system in humans: pathophysiologic implications for primary headache syndromes of the neural influences on the cerebral circulation. J Cereb Blood Flow Metab 1999;19:115–27. 13 Kuritzky A. Cluster headache like syndrome caused by an upper cervical meningioma. Cephalalgia 1984;4:185–6. 14 de la Sayette V, SchaeVer S, Coskun O, et al. Cluster headache-like attack as an opening symptom of a unilateral infarction of the cervical cord; persistent anaesthesia and dysaethesia to cold stimuli. J Neurol Neurosurg Psychiatry 1999;66:397–400. www.jnnp.com 15 Cid C, Berciano J, Pascual J. Retro-ocular headache with autonomic features resembling “continuous” cluster headache in lateral medullary infarction. J Neurol Neurosurg Psychiatry 2000;69:134–4. 16 Hoskin KL, Zagami A, Goadsby PJ. Stimulation of the middle meningeal artery leads to bilateral fos expression in the trigeminocervical nucleus: a comparative study of monkey and cat. J Anat 1999;194:579–88. 17 Goadsby PJ, Knight YE, Hoskin KL. Stimulation of the greater occipital nerve increases metabolic activity in the trigeminal nucleus caudalis and cervical dorsal horn of the cat. Pain 1997;73:23–8. 18 Vincent MB, Ekman R, Edvinsson L, et al. Reduction of calcitonin gene-related peptide in jugular blood following electrical-stimulation of rat greater occipital nerve. Cephalalgia 1992;12:275–9. 19 Nappi G, Micieli G, Cavallini A, et al. Accompanying symptoms of cluster attacks: their relevance to the diagnosticcriteria. Cephalalgia 1992;12:165–8. 20 Bavinzski G, Killer M, Gruber A, et al. Treatment of basilar artery bifurcation aneurysms by using Guglielmi detachable coils: a 6-year experience. J Neurosurg 1999;90:843–52. 21 Byrne JV, Sohn NJ, Molyneux AJ. Five-year experience in using coil embolization for ruptured intracranial aneurysms: outcomes and incidence of late rebleeding. J Neurosurg 1999;90:656–63. Downloaded from http://jnnp.bmj.com/ on October 13, 2016 - Published by group.bmj.com Basilar artery aneurysm with autonomic features: an interesting pathophysiological problem N J Giffin and P J Goadsby J Neurol Neurosurg Psychiatry 2001 71: 805-808 doi: 10.1136/jnnp.71.6.805 Updated information and services can be found at: http://jnnp.bmj.com/content/71/6/805 These include: References Email alerting service Topic Collections This article cites 17 articles, 9 of which you can access for free at: http://jnnp.bmj.com/content/71/6/805#BIBL Receive free email alerts when new articles cite this article. Sign up in the box at the top right corner of the online article. Articles on similar topics can be found in the following collections Headache (including migraine) (427) Pain (neurology) (712) Radiology (1689) Radiology (diagnostics) (1273) Notes To request permissions go to: http://group.bmj.com/group/rights-licensing/permissions To order reprints go to: http://journals.bmj.com/cgi/reprintform To subscribe to BMJ go to: http://group.bmj.com/subscribe/