* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Document 7911466
Electrocardiography wikipedia , lookup
Heart failure wikipedia , lookup
Cardiac contractility modulation wikipedia , lookup
Coronary artery disease wikipedia , lookup
Marfan syndrome wikipedia , lookup
Hypertrophic cardiomyopathy wikipedia , lookup
Myocardial infarction wikipedia , lookup
Turner syndrome wikipedia , lookup
Aortic stenosis wikipedia , lookup
Management of acute coronary syndrome wikipedia , lookup
Arrhythmogenic right ventricular dysplasia wikipedia , lookup
Mitral insufficiency wikipedia , lookup
Cardiothoracic surgery wikipedia , lookup
Lutembacher's syndrome wikipedia , lookup
Atrial septal defect wikipedia , lookup
Quantium Medical Cardiac Output wikipedia , lookup
Dextro-Transposition of the great arteries wikipedia , lookup
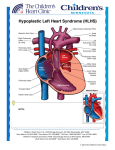
MHBD054-CH68[1231-1240].qxd 11/10/06 13:13 Page 1231 p-mac292 27A:MHBD054:Chapters:CH-68: TechBooks CHAPTER 68 HYPOPLASTIC LEFT HEART SYNDROME Peter J. Gruber, Thomas L. Spray DEFINITION Hypoplastic left heart syndrome includes a wide spectrum of anatomic abnormalities with the common feature of left ventricular hypoplasia and hypoplasia of the ascending aorta. At one end of the spectrum there may be some mild left ventricle hypoplasia, mild aortic stenosis, and aortic coarctation. At the other end of the spectrum, however, there is complete absence of the left ventricle, aortic atresia, or an association with an interrupted aortic arch. All these neonates present a serious challenge to congenital heart surgeons, cardiologists, and their families; the condition is uniformly fatal in the absence of intervention. There is now little argument about the utility of staged operative repair in these children as a result of continued improvements in operative techniques and perioperative care. HISTORICAL HIGHLIGHTS Therapy for HLHS has been one of the great successes of treatment of congenital heart disease (CHD). Before KEY CONCEPTS ● ● ● Epidemiology ● Hypoplastic left heart syndrome (HLHS) accounts for 5 percent of all congenital heart anomalies and is responsible for 25 percent of cardiac deaths in the first week of life. Its incidence is 1.8 in 10,000 live births, with 25 percent of cases showing associated noncardiac malformations and 5 percent showing chromosomal abnormalities. Morphology ● HLHS includes a wide spectrum of anatomic abnormalities with the common feature of hypoplasia of the left ventricle (LV) and the ascending aorta. At one end of the spectrum there may be some mild LV hypoplasia, mild aortic stenosis, and aortic coarctation. At the other end there is complete absence of the LV, aortic atresia, and aortic arch interruption. Pathophysiology ● Systemic venous return is channeled via an interatrial communication to the right ventricle (RV) and, through the pulmonary artery and patent ductus arteriosus (PDA), to the systemic and pulmonary circulations. Balanced physiology ensues, with QP:QS ● ● ● 1231 varying according to systemic and pulmonary vascular resistance as well as the unrestrictive nature of an atrial septal defect (ASD). Clinical features ● HLHS is uniformly fatal if it is not treated as a result of ductal closure in the postnatal period. Evidence of pulmonary overcirculation is provided by QP:QS on clinical examination. Diagnosis ● Prenatal echocardiography can be used to detect unbalanced ventricles as early as 20 weeks of gestational age. Postnatal echocardiography readily establishes the diagnosis and guides medical and surgical decision making. Treatment ● Prostaglandin E1 (PGE1) infusion is the cornerstone of early resuscitation, coupled with balloon atrial septostomy in case of restrictive ASD. A three-stage single-ventricle palliation approach has been adopted by most centers. The first stage involves aortic arch reconstruction and establishment of a reliable source of pulmonary blood flow, and the MHBD054-CH68[1231-1240].qxd 11/10/06 13:13 Page 1232 p-mac292 27A:MHBD054:Chapters:CH-68: TechBooks 1232 ● PART III ● CONGENITAL CARDIAC SURGERY second and third stages consist of sequential partitioning of the systemic and pulmonary circulations. Transplantation has been used as the initial approach or, more commonly, for those who fail palliative strategy. In high-risk neonates, initial palliation with a hybrid approach (stenting of coarctation and pulmonary arterial banding) should be considered. Outcomes ● Operative survival of stage I Norwood palliation depends on several variables, including anatomic the 1980s, HLHS was a uniformly lethal condition. Over the last 25 years, palliation of HLHS has become a standard operation in nearly all institutions. In 1952, Lev1 first described maldevelopment of the left-sided cardiac structures in combination with a small ascending aorta and transverse arch. By 1958, Noonan and Nadas2 had defined the syndrome further to describe a variety of cardiac malformations of left heart structures. The first report of any attempt to palliate a patient with mitral atresia was by Redo and associates,3 who in 1961 performed an atrial septectomy, using inflow occlusion through a right thoracotomy; the patient died soon after the operation. In 1968, Sinha and coworkers4 outlined management principles that remain in use today that include creation of an unobstructed atrial communication, unrestricted ductal flow, and control of pulmonary blood flow. Cayler and colleagues5 described an anastomosis between the right pulmonary artery (PA) and the ascending aorta with banding of both right and left pulmonary arteries. Interestingly, 35 years later, PA banding is being employed in certain centers for selected children who present with a medical or anatomic situation that is not suitable for stage 1 Norwood reconstruction; this first-stage hybrid procedure involves stenting the ductus arteriosus and atrial septal defect and bilateral PA bands.6,7 Litwin, Mohri, and others all performed operations that were variations of these principles that were unsuccessful but contributed to the development of the knowledge of the disease and its palliation.8,9 In 1977, Doty and Knott10 described primary reconstruction that included atrial septation and a right atrium (RA) to PA Fontan circuit. Again, though no patients survived, this experience confirmed that one-stage reconstruction would not be successful because of high neonatal pulmonary vascular resistance (PVR).10 Levitsky, Behrendt, and others described multiple possible surgical procedures; although the procedures demonstrated no longterm success,11,12 they established the principle of staged reconstruction as first described for tricuspid atresia by Fontan and Kreutzer, with a strategy of initial palliation followed by later separation of the systemic and pulmonary circulations.13,14 However, it was Norwood who provided the seminal contribution as the first to palliate infants successfully in 1980 and in 1983 with the first report of a successful staged approach.15,16 The Norwood factors (diameter of ascending aorta, operative weight, restrictive ASD, associated anomalies), and nears 80 percent in several series. Operative mortality between 5 and 10 percent has been observed for second- and third-stage palliation. Long-term complications [ventricular failure, atrioventricular (AV) valve regurgitation, arrhythmias, and protein-loosing enteropathy, among others] dictate a late prognosis and the need for reintervention or transplantation. procedure remains the primary reconstructive approach to this day. EMBRYOLOGY AND ANATOMIC CONSIDERATIONS From a molecular standpoint, the developmental mechanisms that underlie HLHS are obscure, as there are no mutations that have been associated robustly with this part of the heart. Despite the existence of rare family clustering of HLHS, linkage analysis has been unproductive.17–20 However, embryologically, there are clues. The severe hypoplasia of left heart structures is probably a consequence of limited flow during development secondary to a primary abnormality of either left ventricular inflow or left ventricular outflow. Primary defects of myocardial growth are unlikely to be a mechanism for this disease, since the myocardium appears normal.21,22 Additionally, in approximately 5 percent of patients with aortic atresia, an unrestrictive ventricular septal defect coexists, and in such cases, there is nearly always normal development of the left ventricle and the mitral valve. Patients can be categorized on the basis of atrioventricular and semilunar valvular morphology into three primary subsets: (1) aortic atresia with mitral atresia (40 percent), (2) aortic stenosis with mitral stenosis (30 percent), and (3) aortic atresia with mitral stenosis (30 percent) (Fig. 68-1). Aortic stenosis with mitral atresia is rare. HLHS variants include a malaligned AV canal, a double-outlet right ventricle with mitral atresia, tricuspid atresia with transposed great arteries, and a univentricular heart with aortic stenosis. There is frequently leftward and posterior deviation of the septal attachment of the septum primum, but this is unlikely to be a common developmental mechanism, since it is seen commonly in other CHD patients. Usually, the superior and inferior venae cavae (SVC and IVC) are connected normally to the right atrium, though in about 15 percent of these patients a left SVC draining to the coronary sinus is present. Other structural abnormalities of the heart are rare, with less than 5 percent of patients demonstrating AV valvular dysplasia. Also rare (less than 5 percent) is an association with abnormalities of pulmonary venous return or aortic arch interruption. Abnormalities in brain MHBD054-CH68[1231-1240].qxd 11/10/06 13:13 Page 1233 p-mac292 27A:MHBD054:Chapters:CH-68: TechBooks Chapter 68 ● Hypoplastic Left Heart Syndrome 1233 of a large number of mutations that affect valvular development. This argues for a complex early multifactorial event or, more likely, a transient early insult. EPIDEMIOLOGY Figure 68-1 Anatomy and physiology of hypoplastic left heart syndrome. Hypoplastic left-sided structures force pulmonary venous blood to flow from the left atrium through the interatrial communication to the right atrium (downward arrow). Blood from the right ventricle flows both to the pulmonary circulation via the pulmonary artery (upward arrow) and to the systemic circulation via the ductus arteriosus. Maintenance of ductal patency is critical for survival. The surgical principles of repair aim at maintaining this stable physiology. (Reprinted with permission from Gruber P, Spray T, et al. Hypoplastic left heart syndrome. In Kaiser LK, Kron IL (eds.), Mastery of cardiothoracic surgery, 2nd ed. Philadelphia: Lippincott Williams & Wilkins, 2006:936.) development increasingly are being associated with children with severe congenital heart disease and may define a high-risk group for operative repair.23 The pulmonary vascular tree also harbors abnormalities with an increase in number of vessels as well as muscularity.24,25 PATHOPHYSIOLOGY A normal fetus has a parallel circulation that adequately supports single-ventricle physiology before birth. Three communications (the ductus venosus, foramen ovale, and ductus arteriosus) shunt oxygenated placental blood largely past the hepatic and pulmonary beds to supply the splanchnic circulation. Hypoplastic left heart syndrome is well supported in this situation, and as a result, it is rarely a cause of fetal demise. Hypoplastic left heart syndrome is probably a secondary result of early obstructive lesions of either mitral or aortic valvular development. This has been supported in animal models of mitral or aortic stenosis with resulting left ventricular hypoplasia.22 However, the primary cause of the obstructive flow lesion that leads secondarily to HLHS is unknown. Interestingly, there are no known genetic animal models that recapitulate HLHS despite the existence Hypoplastic left heart syndrome is a uniformly fatal disease if it is not treated. It represents 5 percent of all cases of CHD and is responsible for nearly 25 percent of cardiac deaths in the first week of life. Among 10,000 live births, approximately 1.8 will be born with HLHS, with a slight male predominance. Of these, 25 percent also will have a noncardiac anomaly, and 5 percent a chromosomal abnormality (most typically trisomy 13, 18, or 21). Syndromic lesions are rare, but among these patients, Turner’s syndrome(monosomy X) is the most common. The recurrence risk is 2.2 percent for one affected sibling and 6 percent for two affected siblings, suggesting a genetic predisposition but arguing against a simple effect. CLINICAL PRESENTATION The presentation of infants with CHD has changed dramatically over the last 10 years. In most large centers, the majority of patients are identified through prenatal echocardiography; this early identification has not been correlated consistently with a better outcome. Although some tachypnea and mild cyanosis may be present, it is not until the ductus arteriosus begins to close that children with HLHS manifest evidence of impaired systemic perfusion with pallor, lethargy, and diminished femoral pulses. Cardiac examination reveals a dominant right ventricular impulse, a single second heart sound, and often a nonspecific soft systolic murmur. Electrocardiographic examination reveals right atrial enlargement and right ventricular hypertrophy. Chest x-ray occasionally shows mild cardiomegaly and increased pulmonary vascular markings. DIAGNOSIS Physical examination of children with HLHS is usually normal. The examination is determined by the underlying anatomy as well as the chronicity of the disease (ductal closure and PVR). Poor perfusion, weak distal pulses that may not be present because of the size of the ductus, acidosis, and a sepsis-like picture may confound the diagnosis. In the absence of risk factors or laboratory findings consistent with sepsis, one should look for left-sided obstructive lesions. There are no specific laboratory indicators of HLHS. These patients usually exhibit normal values. With ductal closure and malperfusion, end-organ compromise may be reflected by altered hepatic and renal function tests. MHBD054-CH68[1231-1240].qxd 11/10/06 13:13 Page 1234 p-mac292 27A:MHBD054:Chapters:CH-68: TechBooks 1234 PART III ● CONGENITAL CARDIAC SURGERY At 20 weeks of gestation, a fetal echocardiogram allows reasonable visualization of the cardiac structures. It is neither feasible nor cost-effective to screen all pregnancies; a selective approach therefore is taken currently in which only mothers at high risk are screened. Frequently, a ventricular size discrepancy is the first hint of impending problems. Certainly, the presence of an intact or restrictive atrial septum should prompt term high-risk delivery in an institution where an urgent postdelivery atrial septectomy can be performed safely and rapidly. The use of prenatal screening improves the prenatal condition of the child but may not translate into an improved outcome (at least in cases of transposition of the great arteries or HLHS).26,27 After delivery, the infant should undergo two-dimensional and Doppler echocardiography to define anatomy to an extent sufficient for medical and surgical decision making. It is important to distinguish HLHS from other diseases that may mimic certain of its features, as their management differs. Chest radiography often demonstrates mild cardiomegaly and excessive pulmonary blood flow. Head ultrasound should be obtained in all patients to rule out intracranial hemorrhage and minimize the risks of heparinization and circulatory arrest. Patients with medical necrotizing enterocolitis should have a 7-day course of intravenous antibiotics before first-stage palliation. MEDICAL THERAPY Regardless of the anatomic subtype, preoperative stabilization is critical to the ultimate outcome of patients with HLHS. Nearly all patients with suspected HLHS are transported to the authors’ center on PGE1 at a dose of 0.01 to 0.025 g/kg/min. Two clinically important dose-dependent side effects of PGE1 are hypotension and apnea, though they are infrequently clinically important. Umbilical arterial and umbilical venous lines are used for monitoring and central access in most patients. Most infants can ventilate with a natural airway and indeed often have more favorable hemodynamics while extubated. Supplemental oxygen generally should be avoided as it will act as a pulmonary vasodilator, decreasing PVR, increasing Qp:Qs, and thus decreasing systemic perfusion. Inotropic support is required in patients who have had a perinatal insult but otherwise is rarely necessary. The goal of these maneuvers is to get the patient to the operating room in as stable condition as possible. SURGICAL THERAPY There are two primary therapies for HLHS: (1) staged reconstructive surgery leading to a modified FontanKreutzer procedure and (2) heart transplantation. Heart transplantation is discussed in detail in Chapter 80; this chapter focuses on staged reconstructive surgery. Over the last 20 years, the Norwood procedure has evolved and become the standard approach in nearly all institutions that care for neonates and infants with HLHS. There are three primary goals to first-stage palliation: (1) establishment of unrestricted interatrial communication to provide complete mixing and avoid pulmonary venous hypertension, (2) establishment of a reliable source of pulmonary blood flow, allowing for the development of pulmonary vasculature and minimization of volume load on the single ventricle, and (3) provision of unobstructed outflow from the ventricle to the systemic circulation. The Children’s Hospital of Philadelphia offers surgical palliation to nearly all patients with HLHS, including very low birth weight infants and those with nonlethal genetic syndromes. Stage I reconstructive procedure Two perfusion techniques can be used for first-stage palliation: deep hypothermic circulatory arrest (DHCA) and selective antegrade continuous cerebral perfusion. At present, there is no consensus on the superiority of one approach over the other, although DHCA is used more commonly. The child is brought to the operating room and ventilated on room air, with care taken to avoid hyperventilation. A full midline sternotomy is performed, and the thymus is removed in its entirety, with care being taken to avoid the phrenic nerves. The pericardium is opened, and an obligatory mediastinal inspection is performed to confirm the echocardiographic findings, especially to identify abnormalities of the aortic arch and coronary arteries. The ascending and descending aorta, head vessels, ductus arteriosus, and pulmonary arteries are mobilized extensively, with care taken to preserve the integrity of the recurrent laryngeal nerve. No attempt is made to dissect the systemic veins. Purse-string sutures are placed in the proximal main pulmonary artery and generously around the right atrial appendage, through which heparin is administered. A previously thawed pulmonary homograft hemiartery patch is trimmed in an extended arrowhead shape and set aside. After the activated clotting time reaches 300 s, the patient is cannulated, with the arterial cannula inserted at the base of the main pulmonary artery and a single venous cannula inserted in the right atrium (Fig. 68-2). Cardiopulmonary bypass is initiated, and tapes are brought down around the branch pulmonary arteries. The patient is cooled to 18C over 15 min, during which time any remaining dissection is performed. A side-biting clamp is placed on the innominate artery, and a Gore-Tex graft (usually 4.0 mm for patients over 3.2 kg) is anastomosed in an end-to-side fashion. The clamp is removed, and flow is assessed. If blood does not flow easily out of the open shunt, the anastomosis should be revised. A large hemoclip is placed gently to occlude the shunt temporarily. On initiation of circulatory arrest, tapes are brought down around the head vessels and a spoon clamp is MHBD054-CH68[1231-1240].qxd 11/10/06 13:13 Page 1235 p-mac292 27A:MHBD054:Chapters:CH-68: TechBooks Chapter 68 ● Hypoplastic Left Heart Syndrome 1235 connection is performed using interrupted fine polypropylene sutures to connect the root of the great vessels. Next, the arch is reconstructed using the homograft patch, carrying this suture line down to complete the DamusKaye-Stansel (DKS) anastomosis proximally (Fig. 68-3). The distal Blalock-Taussig shunt anastomosis is performed Figure 68-2 Incisions for hypoplastic left heart syndrome first-stage palliation. Cannulation is performed with an arterial cannula in the base of the main pulmonary artery and a single venous cannula through a generous purse string around the right atrial appendage. Subsequent incisions, as described in the text, are indicated by the dotted lines. The patch for aortic augmentation and completion of the aortopulmonary anastomosis is fashioned from a pulmonary homograft (insert). (Reprinted with permission from Gruber P, Spray T, et al. Hypoplastic left heart syndrome. In Kaiser LK, Kron IL (eds.), Mastery of cardiothoracic surgery, 2nd ed. Philadelphia: Lippincott Williams & Wilkins, 2006:938.) placed on the descending aorta distal to the ductal insertion site. Cardioplegia is administered retrograde through a side port on the arterial cannula. After the patient’s blood volume has been drained into the reservoir, all cannulas and PA tapes are removed. The ductus arteriosus is ligated on the PA side and divided on the aortic side. The atrial septum is excised completely, working through the atrial purse-string sutures. Though this is seldom necessary, visualization can be improved through a right atriotomy. Next, the main PA is divided close to the branch PAs, and the defect in the distal main PA segment is closed with an oval homograft patch or primarily, in a vertical fashion. At a point beginning immediately adjacent to the divided main pulmonary artery (MPA) the diminutive aorta is incised medially and the aortotomy is carried superiorly along the underside of the transverse arch through the ductal insertion site to a point approximately 1 cm distal to it. Importantly, all redundant ductal tissue is excised and the coarctation shelf is debrided (alternatively, the segment is excised and the isthmus and proximal descending aorta are reanastomosed). The proximal-aortic-to-proximal-PA A B Figure 68-3 Damus-Kaye-Stansel anastomosis (DKS) and aortic arch reconstruction. After complete excision of ductal tissue and an interrupted anastomosis of the medial aspects of the aorta and the main pulmonary artery, the homograft patch is used to augment the hypoplastic aortic arch and complete the DKS. The modified right Blalock-Taussig shunt is completed from the innominate artery to the right pulmonary artery (A). An alternative approach (Sano modification, B) substitutes a right ventricle–pulmonary artery Gore-Tex conduit for the Blalock-Taussig shunt. (Reprinted with permission from Gruber P, Spray T, et al. Hypoplastic left heart syndrome. In Kaiser LK, Kron IL (eds.), Mastery of cardiothoracic surgery, 2nd ed. Philadelphia: Lippincott Williams & Wilkins, 2006:938–939.) MHBD054-CH68[1231-1240].qxd 11/10/06 13:13 Page 1236 p-mac292 27A:MHBD054:Chapters:CH-68: TechBooks 1236 PART III ● CONGENITAL CARDIAC SURGERY to the origin of the right PA, although some prefer to do this with rewarming after removal of the aortic crossclamp. The arch is infused with cold saline to assess the geometry and rule out kinking or residual obstruction, the atrium is infused with cold saline to de-air, and the cannulas are replaced. Cardiopulmonary bypass is begun, and the patient is warmed to 37C over 22 min. It is important at this point to assess prompt and equivalent filling of the coronary distributions, and any perfusion defect should be addressed immediately with revision of the aortopulmonary anastomosis. During warming, obvious bleeding should be controlled. The average duration of hospitalization is 7 to 21 days, with the limiting factor often being the establishment of adequate oral intake of formula. A Stage II procedure Two important observations by Norwood and colleagues early in the reconstructive experience prompted the institution of an intermediate stage between the initial palliation and the completion of a total cavopulmonary connection. The first observation was that there was timerelated interstage mortality; the second was that the chronic volume load of a systemic-to-pulmonary shunt could create diastolic ventricular dysfunction. Thus, an intermediate stage was initiated as either a bidirectional cavopulmonary (Glenn) shunt or a hemi-Fontan procedure (see Chapter 69). A bidirectional cavopulmonary anastomosis sets the patient up for an extracardiac conduit, whereas a hemi-Fontan sets the patient up for a lateral tunnel completion Fontan. No long-term data prove the superiority of one approach over the other. In general, at approximately 4 to 6 months of age, stage I survivors are catheterized to evaluate both pressures throughout the heart and the anatomy of the pulmonary arteries. The use of a cavopulmonary anastomosis before 3 months of age usually is associated with increased hypoxia and upper body venous congestion. The technique for a bidirectional cavopulmonary anastomosis is fairly standard (Fig. 68-4A). The approach is through a reoperative median sternotomy during which care is taken in regard to the dissection of the neoaorta, which is often adherent to the left side of the sternum and somewhat fragile. The patient is cannulated in a standard fashion with an arterial cannula in the neoaorta, a small angled venous cannula in the SVC, and a straight cannula in the body of the RA. Although it is possible to perform this procedure without cardiopulmonary bypass (CPB), the authors find it considerably easier to use CPB and believe that the anatomic result is improved. Extracorporeal circulation is begun, and a tape is brought down around the SVC cannula after ligation and division of the azygous vein. The previous shunt is divided and ligated near the innominate artery. A vascular clamp is placed at the SVC-RA junction, and the SVC is divided. The atrial portion is closed in two layers with fine monofilament sutures. The PA is opened in the superior portion and inspected carefully. If preoperative B Figure 68-4 Superior cavopulmonary anastomosis. A. The bidirectional Glenn replaces the source of obligatory pulmonary blood flow from the Blalock-Taussig shunt to the superior vena cava. B. The hemi-Fontan results in precisely the same physiology but uses a single piece of homograft to augment the pulmonary arteries, complete the superior cavopulmonary anastomosis, and create the right atrium–pulmonary artery dam simultaneously. (Reprinted with permission from Gruber P, Spray T, et al. Hypoplastic left heart syndrome. In Kaiser LK, Kron IL (eds.), Mastery of cardiothoracic surgery, 2nd ed. Philadelphia: Lippincott Williams & Wilkins, 2006:940–941.) catheterization revealed pulmonary arterial stenosis, this is addressed with pulmonary homograft patch augmentation. The SVC then is anastomosed to the superior aspect of the right pulmonary artery as medially as possible toward the left PA. The tape is removed from the SVC cannula, and one or two right atrial lines are brought into the mediastinum percutaneously from the right and placed in the body of the right atrium. The patient is weaned from CPB, and the SVC cannula is removed. Modified ultrafiltration is performed, and during that MHBD054-CH68[1231-1240].qxd 11/10/06 13:13 Page 1237 p-mac292 27A:MHBD054:Chapters:CH-68: TechBooks Chapter 68 ● Hypoplastic Left Heart Syndrome time, all suture lines are checked for hemostasis. At the completion of modified ultrafiltration, the SVC pressure can be measured directly. The SVC monitoring line is removed, and the cannulation purse string is tied securely. All cannulas are removed, and protamine is administered. The chest is closed in a standard fashion, and the patient is returned to the intensive care unit (ICU). In general, these patients can be extubated soon after their return to the ICU. An alternative operative approach is the hemi-Fontan, which the authors perform under deep hypothermic circulatory arrest. The details of this procedure are summarized in Fig. 68-4B and are described in Chapter 69. The hemi-Fontan is performed under DHCA, using a piece of folded homograft to augment the pulmonary arteries and create the superior cavopulmonary anastomosis and the RA-PA dam simultaneously. This dam subsequently will be removed for the lateral tunnel completion Fontan operation. 1237 aspect of the pulmonary arteries, angled slightly medially to the SVC. Practically, the angled nature of the superior portion of the conduit augments the pulmonary confluence from the right to the left PA (Fig. 68-5A). The conduit is infused with saline to de-air, and the patient is weaned off CPB. The SVC cannula is removed, and a period of modified ultrafiltration is begun. All suture Stage III procedure Between ages 2 and 5, usually on the basis of a combination of the patient’s weight, growth characteristics, and arterial saturations, the patient is reimaged by echocardiography and occasionally with cardiac catheterization. If there are no anatomic issues to be addressed with catheter-based intervention (e.g., distal arch coarctation), the patient is referred for Fontan reconstruction via an extracardiac conduit or a lateral tunnel completion Fontan. For the extracardiac conduit, DHCA or continuous bypass with or without aortic cross-clamping is employed, whereas DHCA is used for the lateral tunnel. The approach is through a reoperative median sternotomy. The following material describes the basic steps of the extracardiac Fontan. The patient is cannulated bicavally in a standard fashion, and an arterial cannula is placed high in the aortic reconstruction. Cardiopulmonary bypass is begun, and tourniquets are applied around the caval cannulas. A vascular clamp is placed at the IVC-RA junction, and the IVC is divided. The atrial portion is closed partially in two layers with fine monofilament sutures. The conduit (18- to 22-mm Gore-Tex) is trimmed to the appropriate length to avoid compression of the right pulmonary vein (which typically is shorter than one might expect), and a 4-mm fenestration is punched in the medial aspect near the IVC portion. The IVC-conduit anastomosis is completed with monofilament sutures, followed by a side-toside anastomosis of the remaining cardiac portion of the IVC opening with the exterior conduit, leaving a rim of conduit around the fenestration. Next, the PAs are opened in the inferior portion and inspected directly. If preoperative studies revealed any pulmonary arterial stenosis and the beveled end of the Gore-Tex conduit will not span the area of stenosis, this situation is addressed with pulmonary homograft patch augmentation. The conduit then is anastomosed to the inferior A B Figure 68-5 Fontan completion. A. The extracardiac Fontan uses a Gore-Tex tube graft to channel inferior vena cava (IVC) blood outside the heart to the pulmonary circuit. A 4-mm fenestration is fashioned between the remnant cardiac portion of the IVC and the side of the conduit. B. The lateral tunnel Fontan baffles blood from the IVC along the lateral aspect of the atrium inside the heart to the pulmonary circuit. The homograft dam created during the hemi-Fontan is excised to create continuity with the pulmonary arteries. Fenestration patency has proved more reliable in this procedure than in the extracardiac Fontan. (Reprinted with permission from Gruber P, Spray T, et al. Hypoplastic left heart syndrome. In Kaiser LK, Kron IL (eds.), Mastery of cardiothoracic surgery, 2nd ed. Philadelphia: Lippincott Williams & Wilkins, 2006:943–944.) MHBD054-CH68[1231-1240].qxd 11/10/06 13:13 Page 1238 p-mac292 27A:MHBD054:Chapters:CH-68: TechBooks 1238 PART III ● CONGENITAL CARDIAC SURGERY lines are checked for hemostasis. At the completion of modified ultrafiltration, the SVC pressure is measured directly with a transthoracic line. An additional monitoring line is placed in the right atrium. All cannulas are removed, and protamine is administered. The chest is closed in a standard fashion, and the patient is returned to the ICU. In general, these patients can be extubated soon after their return to the ICU. An alternative operative approach is the lateral tunnel Fontan, which the authors perform under deep hypothermic circulatory arrest. The technical details of this procedure are summarized in Fig. 68-5B. The lateral tunnel Fontan is performed under DHCA, using a piece of fenestrated Gore-Tex patch to baffle blood from the IVC to the pulmonary arteries. The previously constructed PA-RA homograft dam is excised. There are no long-term data suggesting the superiority of one technique over the other, but the lack of an atrial suture line with the extracardiac Fontan has led to a preference for its use when extensive PA reconstruction is necessary. OUTCOMES Despite continued improvement in outcomes, patients with HLHS continue to present a formidable challenge. The authors’ group reported the results of the 15-year Children’s Hospital of Philadelphia (CHOP) experience with 840 patients undergoing stage I surgery for HLHS between 1984 and 1999. The 1-, 2-, 5-, 10-, and 15year survivals for the entire cohort were 51 percent, 43 percent, 40 percent, 39 percent, and 39 percent, respectively. A later era of stage I palliation was associated with significantly improved survival, with 3-year survival for patients undergoing stage I reconstruction from 1995 to 1998 of 66 percent versus 28 percent for those undergoing surgery between 1984 and 1988.28 More recent studies have shown further improvement and demonstrated that the variability in outcome is influenced by anatomy. One retrospective study of risk factors for operative and 1-year mortality in 158 patients who underwent the Norwood procedure demonstrated that the diagnosis of HLHS is not a predictor of mortality. HLHS was present in 102 patients, and other forms of functional single ventricle with systemic outflow tract obstruction were observed in the remaining 56. Operative survival for the entire cohort was 77 percent (78 percent for patients with HLHS and 75 percent for patients with other diagnoses). Birth weight, associated cardiac anomalies, total support time, and the need for extracorporeal membrane oxygenation (ECMO) or ventricular assist device (VAD) support were predictors of operative mortality. Survival to 1 year was 86 percent after successful first-stage palliation, although the presence of an extracardiac anomaly, a genetic syndrome, or an additional cardiac defect was predictive of worse survival in the first year of life.29 Additional perioperative or operative treatment strategies may improve morbidity and mortality rates. A consecutive series of 115 patients who underwent stage I palliation from Milwaukee identified the risk factors for mortality and the impact of new treatment strategies. Between 1996 and 2001, hospital survival was 93 percent compared with 53 percent for the time interval between 1992 and 1996. Survival to stage II palliation also was improved significantly, increasing from 44 percent to 81 percent. Anti-inflammatory treatment strategies, continuous saturated venous oxygen (Svo2) monitoring, and the use of phenoxybenzamine were factors favoring survival to stage II.30 Another approach has been the development of home surveillance programs. One study compared patients discharged before the initiation of home surveillance with those discharged with an infant scale and a pulse oximeter. The parents maintained a daily log of weight and arterial oxygen saturation according to pulse oximetry and were instructed to contact their physicians in case of arterial oxygen saturation less than 70 percent according to pulse oximetry, an acute weight loss of more than 30 g/24 h, or failure to gain at least 20 g during a 3-day period. Interstage mortality was 15.8 percent in the historic group and 0 percent in the monitored group, suggesting that monitoring programs may provide significant benefit in reducing interstage attrition.31 Recent reports advocate that an RV-PA conduit (rather than a modified Blalock-Taussig shunt) as reintroduced by Sano and colleagues may improve the outcome after stage I reconstruction.32–34 However, further studies have indicated the need for caution before broad adoption of the RV-PA conduit. The CHOP group compared the outcomes of all neonates who underwent a stage I reconstruction between 2002 and 2004 with the use of the RV-PA conduit and modified Blalock-Taussig shunt. In all, 149 infants underwent a stage I reconstruction for HLHS or its variants. There was no difference in surgical mortality, time to extubation, or length of hospital stay between the two techniques. Although there was no difference in overall mortality, patients with an RVPA conduit required more conduit-related reinterventions and returned earlier for stage II reconstruction.35 Patients who have completed staged palliation for HLHS frequently require reintervention. A study examining these reinterventions during a 6-year period between 1995 and 2001 found that 123 procedures were performed in 71 patients. The median time from Fontan completion to reoperation was 3.6 years, with indications for reintervention including arrhythmia, cyanosis, exercise intolerance, protein-losing enteropathy (PLE), atrioventricular valve (AVV) regurgitation, and other indications. The procedures included pacemaker insertion or revision (48 percent), reinclusion of previously excluded hepatic veins (13 percent), revision to a lateral tunnel or extracardiac conduit pathway (11 percent), cardiac transplantation (7 percent), enlargement or creation MHBD054-CH68[1231-1240].qxd 11/10/06 13:13 Page 1239 p-mac292 27A:MHBD054:Chapters:CH-68: TechBooks Chapter 68 ● Hypoplastic Left Heart Syndrome of a baffle fenestration (5 percent), isolated AVV repair or replacement (2 percent), and other procedures (14 percent). There were five early and five late deaths. Hospital mortality was greatest among patients undergoing cardiac transplantation (44 percent), who accounted for 80 percent of the early deaths. Most reinterventions can be performed with minimal morbidity and mortality. However, patients who require cardiac transplantation after a Fontan procedure fare poorly.36 For high-risk infants, some have advocated catheterbased hybrid approaches (stage I: ductal stenting and PA banding; stage II: septectomy, arch augmentation, and cavopulmonary anastomosis; stage III: catheter-based Fontan completion). One report of the hybrid stage I procedure in 14 high-risk neonates between 2003 and 2005 demonstrated promising results, with a 78.5 percent hospital survival rate. Eight patients underwent stage II procedures, with two operative deaths. These techniques may be limited in certain anatomic subsets, such as patients with aortic atresia in which preductal retrograde coarctation is a significant problem.6,37 The results from stage III palliation with Fontan circulation also continue to improve. The authors’ group reviewed the outcomes of 332 patients: 281 with a lateral tunnel and 51 with an extracardiac Fontan procedure. There was a 93.4 percent hospital survival rate, and by questionnaire, 94.6 percent of guardians described their children’s overall health as excellent or good and 5.4 percent reported it as fair or poor. School performance was described as above average in 30.2 percent, average in 39.9 percent, and below average in 29.8 percent. With regard to cardiac functional status, 34.2 percent responded that their children had no limitations to physical activity, 52.5 percent reported a slight limitation, 12.1 percent reported a significant limitation, and 1.2 percent reported a severe limitation. Although these data suggest that acceptable survival outcomes have been 1239 observed at intermediate follow-up of the Fontan operation, much work remains to be done.38 In comparing outcomes of extracardiac conduit and lateral tunnel Fontan connections, the results have been conflicting. The Toronto group reported the results of 60 extracardiac conduit and 47 lateral tunnel total cavopulmonary connections performed between 1994 and 1998. Overall operative mortality was 5.6 percent and did not differ between groups. The lateral tunnel group had a significantly higher incidence of postoperative sinoatrial node dysfunction, supraventricular tachycardia, and need for temporary postoperative pacing. The median duration of ICU stay and the need for ventilatory support were longer in the lateral tunnel group. Holter analysis showed a higher incidence of atrial arrhythmias in the lateral tunnel group.39 However, other authors have not found support for one technique over the other. For example, the Charleston group found an incidence of sinus node dysfunction of 21 percent in the lateral tunnel group and 59 percent in the extracardiac group. No permanent pacemaker was placed in the lateral tunnel group, whereas three were placed in the extracardiac group group.40 Another report from that group demonstrated identical operative mortality and similar mean Fontan pressure, transpulmonary gradient, and common atrial pressure in the first 24 h after the completion of a cavopulmonary connection. Duration of mechanical ventilation, ICU stay, chest tube drainage, and hospital stay did not differ. Again, extracardiac conduit patients had a higher incidence of sinus node dysfunction both in the postoperative period and at hospital discharge.41 These techniques also appear equivalent in the situation of a failing Fontan, with no hospital deaths and no arrhythmias at hospital discharge or differences in mean duration of intubation, inotropic support, ICU stay, hospital stay, or episodes of acute postoperative arrhythmias.42 References 1. Lev M. Pathologic anatomy and interrelationship of hypoplasia of the aortic tract complexes. Lab Invest 1952;1:61. 2. Noonan JA, Nadas AS. The hypoplastic left heart syndrome: An analysis of 101 cases. Pediatr Clin North Am 1958;5:1029. 3. Redo SF, Farber S, Gross RE. Atresia of the mitral valve. Arch Surg 1961;82:696. 4. Sinha SN, Rusnak SL, Sommers HM, et al. Hypoplastic left ventricle syndrome: Analysis of thirty autopsy cases in infants with surgical considerations. Am J Cardiol 1968; 21:166. 5. Cayler GG, Smeloff EA, Miller GE Jr. Surgical palliation of hypoplastic left side of the heart. N Engl J Med 1970; 282:780. 6. Bacha EA, Daves S, Hardin J, et al. Single-ventricle palliation for high-risk neonates: The emergence of an alterna- tive hybrid stage I strategy. J Thorac Cardiovasc Surg 2006;131:163. 7. Pizarro C, Norwood WI. Pulmonary artery banding before Norwood procedure. Ann Thorac Surg 2003;75: 1008. 8. Litwin SB, Friedberg DZ. Surgical management of a neonate with interrupted aortic arch, transposition of the great Arteries, and tricuspid atresia. Cardiovasc Dis 1975; 2:182. 9. Mohri H, Horiuchi T, Haneda K, et al. Surgical treatment for hypoplastic left heart syndrome: Case reports. J Thorac Cardiovasc Surg 1979;78:223. 10. Doty DB, Knott HW. Hypoplastic left heart syndrome: Experience with an operation to establish functionally normal circulation. J Thorac Cardiovasc Surg 1977;74:624. 11. Behrendt DM, Rocchini A. An operation for the hypoplastic left heart syndrome: Preliminary report. Ann Thorac Surg 1981;32:284. MHBD054-CH68[1231-1240].qxd 11/10/06 13:13 Page 1240 p-mac292 27A:MHBD054:Chapters:CH-68: TechBooks 1240 PART III ● CONGENITAL CARDIAC SURGERY 12. Levitsky S, van der Horst RL, Hasteiter AR, et al. Surgical palliation in aortic atresia. J Thorac Cardiovasc Surg 1980;79:456. 13. Fontan F, Baudet E. Surgical repair of tricuspid atresia. Thorax 1971;26:240. 14. Kreutzer G, Galindez E, Bono H, et al. An operation for the correction of tricuspid atresia. J Thorac Cardiovasc Surg 1973;66:613. 15. Norwood WI, Kirklin JK, Sanders SP. Hypoplastic left heart syndrome: Experience with palliative surgery. Am J Cardiol 1980;45:87. 16. Norwood WI, Lang P, Hansen DD. Physiologic repair of aortic atresia-hypoplastic left heart syndrome. N Engl J Med 1983;308:23. 17. Gelb BD. Molecular genetics of congenital heart disease. Curr Opin Cardiol 1997;12:321. 18. Loffredo CA, Chokkalingam A, Sill AM, et al. Prevalence of congenital cardiovascular malformations among relatives of infants with hypoplastic left heart, coarctation of the aorta, and d-transposition of the great arteries. Am J Med Genet A 2004;124:225. 19. McBride KL, Fernbach S, Menesses A, et al. A familybased association study of congenital left-sided heart malformations and 5,10 methylenetetrahydrofolate reductase. Birth Defects Res A Clin Mol Teratol 2004; 70:825. 20. McElhinney DB, Krantz ID, Bason L, et al. Analysis of cardiovascular phenotype and genotype-phenotype correlation in individuals with a JAG1 mutation and/or Alagille syndrome. Circulation 2002;106:2567. 21. Chen B, Bronson RT, Klaman LD, et al. Mice mutant for Egfr and Shp2 have defective cardiac semilunar valvulogenesis. Nat Genet 2000;24:296. 22. Sedmera D, Hu N, Weiss KM, et al. Cellular changes in experimental left heart hypoplasia. Anat Rec 2002;267: 137. 23. Forbess JM, Visconti KJ, Bellinger DC, et al. Neurodevelopmental outcomes in children after the Fontan operation. Circulation 2001;104:I127. 24. Macedo A, Pinto E, Ramos S, et al. Structural changes in pulmonary vessels and coronary arteries in hypoplastic left heart syndrome. Acta Med Port 1991;4:253. 25. Maeda K, Yamaki S, Kado H, et al. Hypoplasia of the small pulmonary arteries in hypoplastic left heart syndrome with restrictive atrial septal defect. Circulation 2004;110:II139. 26. Kumar RK, Newburger JW, Gauvreau K, et al. Comparison of outcome when hypoplastic left heart syndrome and transposition of the great arteries are diagnosed prenatally versus when diagnosis of these two conditions is made only postnatally. Am J Cardiol 1999;83:1649. 27. Satomi G, Yasukochi S, Shimizu T, et al. Has fetal echocardiography improved the prognosis of congenital heart disease? Comparison of patients with hypoplastic left heart syndrome with and without prenatal diagnosis. Pediatr Int 1999;41:728. 28. Mahle WT, Spray TL, Wernovsky G, et al. Survival after reconstructive surgery for hypoplastic left heart syndrome: A 15-year experience from a single institution. Circulation 2000;102:III136. 29. Gaynor JW, Mahle WT, Cohen MI, et al. Risk factors for mortality after the Norwood procedure. Eur J Cardiothorac Surg 2002;22:82. 30. Tweddell JS, Hoffman GM, Mussatto KA, et al. Improved survival of patients undergoing palliation of hypoplastic left heart syndrome: Lessons learned from 115 consecutive patients. Circulation 2002;106:I82. 31. Ghanayem NS, Hoffman GM, Mussatto KA, et al. Home surveillance program prevents interstage mortality after the Norwood procedure. J Thorac Cardiovasc Surg 2003;126: 1367. 32. Sano S, Ishino K, Kado H, et al. Outcome of right ventricle-to-pulmonary artery shunt in first-stage palliation of hypoplastic left heart syndrome: A multi-institutional study. Ann Thorac Surg 2004;78:1951. 33. Sano S, Ishino K, Kawada M, et al. Right ventriclepulmonary artery shunt in first-stage palliation of hypoplastic left heart syndrome. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu 2004;7:22. 34. Pizarro C, Malec E, Maher KO, et al. Right ventricle to pulmonary artery conduit improves outcome after stage I Norwood for hypoplastic left heart syndrome. Circulation 2003;108(Suppl 1):II155. 35. Tabbutt S, Dominguez TE, Ravishankar C, et al. Outcomes after the stage I reconstruction comparing the right ventricular to pulmonary artery conduit with the modified Blalock Taussig shunt. Ann Thorac Surg 2005; 80:1582. 36. Petko M, Myung RJ, Wernovsky G, et al. Surgical reinterventions following the Fontan procedure. Eur J Cardiothorac Surg 2003;24:255. 37. Galantowicz M, Cheatham JP. Lessons learned from the development of a new hybrid strategy for the management of hypoplastic left heart syndrome. Pediatr Cardiol 2005; 26:190. 38. Mitchell ME, Ittenbach RF, Gaynor JW, et al. Intermediate outcomes after the Fontan procedure in the current era. J Thorac Cardiovasc Surg 2006;131:172. 39. Azakie A, McCrindle BW, Van Arsdell G, et al. Extracardiac conduit versus lateral tunnel cavopulmonary connections at a single institution: Impact on outcomes. J Thorac Cardiovasc Surg 2001;122:1219. 40. Dilawar M, Bradley SM, Saul JP, et al. Sinus node dysfunction after intraatrial lateral tunnel and extracardiac conduit Fontan procedures. Pediatr Cardiol 2003;24:284. 41. Kumar SP, Rubinstein CS, Simsic JM, et al. Lateral tunnel versus extracardiac conduit Fontan procedure: A concurrent comparison. Ann Thorac Surg 2003;76:1389. 42. Morales DL, Dibardino DJ, Braud BE, et al. Salvaging the failing Fontan: Lateral tunnel versus extracardiac conduit. Ann Thorac Surg 2005;80:1445.