* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Diagnosis and Treatment of Constrictive Pericarditis
Survey
Document related concepts
Remote ischemic conditioning wikipedia , lookup
Heart failure wikipedia , lookup
Electrocardiography wikipedia , lookup
Coronary artery disease wikipedia , lookup
Cardiac contractility modulation wikipedia , lookup
Cardiac surgery wikipedia , lookup
Lutembacher's syndrome wikipedia , lookup
Myocardial infarction wikipedia , lookup
Management of acute coronary syndrome wikipedia , lookup
Jatene procedure wikipedia , lookup
Mitral insufficiency wikipedia , lookup
Dextro-Transposition of the great arteries wikipedia , lookup
Hypertrophic cardiomyopathy wikipedia , lookup
Arrhythmogenic right ventricular dysplasia wikipedia , lookup
Transcript
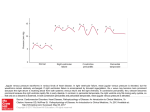
Diagnosis and Treatment of Constrictive Pericarditis Edwin G. Avery, IV, M.D. Chief, Division of Cardiac Anesthesia University Hospitals Case Medical Center Associate Professor of Anesthesiology Case Western Reserve Univ. School of Medicine Objectives: I. 1) 2) 3) 36th SCA Annual Meeting & Workshops March 29 – April 2, 2014 New Orleans, LA Develop a basic understanding of diastolic cardiac function as assessed by cardiac ultrasound exam Understand the difference between constrictive and restrictive cardiac pathologies Be aware of the most effective diagnostic modalities employed to determine the need for pericardiectomy Pathophysiology of Constrictive Pericarditis Constrictive pericarditis (CP) represents the final stages in the progression of an inflammatory process resulting from a wide range of possible pathologies affecting the pericardium (e.g., idiopathic, postsurgical/trauma, infectious, autoimmune disorders, irradiation, uremia, neoplastic, sarcoid, methysergide therapy). The end result is that the heart’s diastolic function (i.e. lusitropism) is limited by constriction related to changes in the pericardium (i.e. thickening, fibrosis and calcification), thus the term constrictive pericarditis. The time course for constriction to occur following an inflammatory insult is variable and may span from months to years. In some cases the fibrosis with associated thickening can develop rapidly and may be reversible (most commonly following cardiac surgery).1 The dense fibrosis that characterizes CP commonly involves adhesions between the visceral and parietal pericardial layers. From a pathophysiologic standpoint the developed constriction affects lusitropism and effectively limits cardiac chamber filling. Elevation of systemic and pulmonary venous pressures further characterizes the pathophysiology. In CP the ventricular chambers are known to fill rapidly in early diastole as a result of the elevated atrial pressures; ventricular filling ceases early and abruptly when the chamber reaches its limited maximum volumetric capacity. The limited filling capacity of the ventricle(s) has different pathologic consequences depending on whether one or both sides of the heart are affected. For example, in an individual with constrictive changes isolated to the right ventricle there will be signs/symptoms of systemic venous congestion (e.g., hepatic congestion with associated coagulopathy and hepatic dysfunction, peripheral edema, ascites, anasarca and possibly hepatic cirrhosis). Alternatively, constrictive changes primarily affecting the left ventricle will result in pulmonary edema/hypertension and respiratory symptoms (e.g., exertional dyspnea, orthopnea and cough) The limited cardiac filling may also result in a compromised stroke volume and thus reduced cardiac output putting all organs at risk for impaired perfusion. In individuals suffering from constrictive changes to both sides of the heart the signs and symptoms will be mixed. Further, the reduced cardiac output may result in physiologic compensatory changes involving the retention of sodium and water by the kidneys that can contribute to elevation of the already high venous pressures. When CP is severe affected individuals may develop atrial fibrillation, atrioventricular regurgitation, severe fatique and cachexia. An important aspect of CP pathophysiology is that the thickened pericardium often insulates the heart from the changes in intrathoracic pressures that result from normal negative pressure respiration. Under normal physiologic conditions the decrease in inspiratory pressure during spontaneous respiration results in augmented filling of the right ventricle and some pooling of blood in the lower compliance pulmonary veins that is associated with decreased filling of the left ventricle. Because the thickened pericardium effectively insulates the heart from these pressures changes ventricular function is further compromised. As one might suspect the changes in ventricular filling in this diastolic disorder can result in readily apparent changes in the movement of the Interventricular septum. In many advanced cases of CP there is a characteristic “septal bounce” observed that can be seen with a number of diastolic disorders, including restrictive pathologies (e.g., hypertrophic cardiomyopathy, sarcoid cardiomyopathy, hypertensive cardiomyopathy). To better understand the physiologic changes associated with CP readers are encouraged to fully review the anatomy and physiology of the pericardium.2,3 II. Diagnosing Constrictive Pericarditis – General Considerations The accurate diagnosis of CP is complicated by its overlap with the clinical signs and symptoms that characterize other cardiac pathologies that primarily stem from diastolic dysfunction (e.g., restrictive cardiomyopathies as referenced in the preceding section). In general while the clinical signs and symptoms may bring the affected patient to seek medical care they are not specific enough to help make the distinction between constrictive and restrictive physiologic disorders. Thus, other more advanced diagnostic methodologies are employed that fall into the realm of diagnostic imaging. The most common diagnostic approach in an individual with apparent signs and symptoms of diastolic dysfunction will include obtaining a chest roentgenogram, an electrocardiogram and a cardiac ultrasound (i.e. transthoracic and/or transesophageal). Avery EG/Pericardial Disease Page 2 of 7 While the chest film and ECG are of minimal help in making this diagnosis the cardiac ultrasound exam, especially transesophageal echocardiography is common practice in making the diagnosis of CP. Cardiac ultrasound accomplishes two essential elements of diagnosing CP; first, to verify the diffuse nature of the presence of pericardial thickening and calcification, and the second, to demonstrate diastolic dysfunction in the setting of normal to near normal systolic function and/or normal appearing myocardium. Higher fidelity imaging studies such as cardiac MRI or 320, or 256 slice computed tomography far outshine transthoracic and even transesophageal echocardiography in quantifying the diffuse nature of the presence of a thickened pericardium (normally the pericardium is 2-3 mm in thickness). Cardiac MRI, also termed cardiovascular magnetic resonance (CMR) or cine-based CT imaging may also reveal the characteristic and nonspecific septal bounce associated with CP and many other forms of diastolic dysfunction. Additionally, CMR is considered the gold standard for volumetric assessment of the left ventricle and can thus most accurately determine stroke volume and cardiac output (i.e. systolic function). Despite the strengths of the higher fidelity, non-ultrasound based imaging modalities, they have historically not been able to provide the same level of physiologic insight into the collective nature of cardiac physiological dysfunction, somewhat related to a lack of temporal resolution capacity. However, recent advances in CMR techniques appears to prove that this technique can also accurately detect and measure the transatrioventricular valvular velocities as well as the transpulmonary venous flow velocities associated with CP and potentially other cardiac pathologies.4 Further, CMR is also able to provide analysis of both mitral annular tissue velocities as well as flow propagation velocities across the mitral valve.5 Considering the widespread use, relative accessibility to, relatively shorter time to complete an exam and lower expense associated with the use of cardiac ultrasound as well as the large body of literature which has accumulated to prove that cardiac ultrasound examination can accurately and reliably diagnose CP it seems apparent for the present that use of cardiac ultrasound will remain the mainstay of diagnosis for this disorder. It is anticipated that as the body of literature related to the use of CMR to diagnose CP grows that it will effectively challenge the use of cardiac ultrasound as the mainstay of diagnosing CP. III. Diagnosing Constrictive Pericarditis – Cardiac Ultrasound Examination Pericarditis is an inflammation of the pericardium and can occur as the result of multiple pathologies. Pericarditis may present as either a chronic or acute process and is often associated with the development of a pericardial effusion. Clinically, one examines the patient suspected of having pericarditis for the presence of the classic triad of chest pain, ECG evidence of pericarditis (e.g., diffuse ST segment elevations), and a pericardial rub on auscultation. Etiologies of pericarditis include the following: Infections (e.g., postviral, bacterial-especially tuberculosis) Neoplastic (e.g., primary-mesothelioma or fibrosarcoma; secondary metastatic disease-melanoma, lymphoma, leukemia or direct extension of a pulmonic or breast tumor) Immune/Inflammatory (e.g., rheumatoid arthritis, systemic lupus erythematosus, scleroderma, acute rheumatic fever, dermatomyositis, Wegener’s granulomatosis, mixed collagen vascular disease; post-myocardial infarction (Dressler’s syndrome); uremia; postcardiac surgery Intracardiac-pericardial communications (e.g., chest trauma, post-interventional catheter procedures, postmyocardial ventricular rupture)2. Echocardiographically, one inspects the pericardium for evidence of thickening that appears as an increase in the brightness, or echogenicity of the ultrasound signal. Normal pericardial thickness is approximately 2-3 mm. M-mode analysis of a patient with pericarditis will reveal multiple parallel ultrasound reflections and can be helpful to discern the thickened pericardium (Figure 1). Multiple echo windows should be obtained to verify the diffuse or localized nature of the pericarditis. Echocardiography lacks sufficient fidelity to consistently quantify the degree of pericarditis and thus carries an unreliable sensitivity and specificity for detecting this disorder compared to other imaging techniques (e.g, cardiac MRI or high resolution (256 or 320 slice) computed tomography). The evaluation of a subtype of pericarditis termed Constrictive pericarditis is important to be aware of when diagnosing pericardial disease. Clinically significant constrictive pericarditis (CP) will affect ventricular filling in that it can mimic restrictive diastolic dysfunction. CP has been reported to occur in 0.2 – 0.3% of cardiac surgical patients and has a number of pathophysiologic features that include: fibrotic pericardium, inflamed pericardium, calcific thickening of the pericardium, abnormal diastolic filling, a narrow RV pulse pressure (i.e. the systolic RV pressure is normal but the diastolic RV pressure is elevated), a prominent early diastolic RV pressure dip and later plateau (termed the “square root sign” (Figure 2)3. Additionally, the RA pressure exhibits a pronounced systolic drop (y descent followed by an increase and then plateau) that appears as an “M-like” wave on the CVP tracing (Figure 2). The diagnosis of CP is made by using a combination of 2D echocardiographic analysis and Doppler analysis. The 2D echocardiographic exam will generally reveal a thickened, highly reflective pericardium in patients with CP. TEE can detect the thickened pericardium but computed tomography and magnetic resonance imaging are considered to provide more accurate Avery EG/Pericardial Disease Page 3 of 7 measurements of the pericardial thickness. Table 1 lists a number of nonspecific echocardiographic findings associated with CP1. Figure 1. M-mode image (the arrows highlight a thickened pericardium). Avery EG & Shernan SK. Comprehensive Textbook of Perioperative Transesophageal Echocardiography 2ed. Chapter 39. 2010 Figure 2 Table 1. 2D-Echocardiographic Findings Associated with CP Paradoxical ventricular septal motion Ventricular septal "bounce" Diastolic flattening of the posterior LV Premature mid-diastolic pulmonary valve opening Spontaneous inspiratory leftward shift of the atrial & ventricular septum Enlarged hepatic veins Dilated IVC without variation in size during respiration Normal ventricular size Normal or enlarged atria with reduced wall excursion Doppler assessment of patients with CP is complex in that multiple Doppler modalities are recommended to accurately diagnose this pathology. Doppler modalities used to assess CP include: pulse wave Doppler transmitral tracings, pulse wave Doppler pulmonary vein tracings, tissue Doppler imaging of the mitral annulus and color Doppler M-mode (flow propagation, Vp) of the transmitral inflow. Transmitral blood flow has proven to be a useful diagnostic and prognostic tool for individuals with CP. By color Doppler assessment patients with CP may exhibit tricuspid or mitral valve incompetence. Similar to pericardial tamponade, the transmitral pulse wave Doppler profile of most patients with CP will demonstrate an exaggerated respirophasic variation (i.e. it will decrease by approximately 25% during spontaneous inspiration). Note that up to 20% of patients with CP will not exhibit this Doppler finding although the application of preload reducing maneuvers (e.g., reverse Trendelenberg position) may be useful to amplify the transmitral velocity respiratory variation that is expected in these patients15. The thickened pericardium insulates the intrapericardial structures from the intrathoracic pressure changes associated with the respiratory cycle and produces an exaggerated respirophasic variation as a result (as seen in tamponade); similar respirophasic variation is observed in the pulmonary veins with CP and the S:D ratios observed are similar to those of patients with restrictive myocardial pathology (e.g., the pulmonary vein diastolic flow velocity will be greater than the systolic flow velocity in patients with a restrictive LV filling pattern, or S:D ratio < 1)3,18-20. Additionally, unique to CP relative to restrictive cardiomyopathy is that the peak amplitude of the pulmonary venous D wave has been noted to exhibit pronounced respiratory variation ( > 18% increase upon inspiration in patients receiving IPPV) in CP20. During mechanical ventilation the transmitral E-wave velocity will increase with early inspiration in CP, thus the exaggerated respirophasic variation seen with spontaneously breathing CP patients is also observed with IPPV, although the direction of change in velocity will be opposite in direction21. The increase in intrathoracic pressure expels blood from the extrapericardial pulmonary veins into the left atrium which results in an increase in transmitral flow velocity. Again the mechanism for the Avery EG/Pericardial Disease Page 4 of 7 exaggerated respirophasic variation observed in CP patients is that the thickened pericardium insulates the intracardiac chambers (the LA in this case) from the changes in intrathoracic pressure thus increasing the gradient between the pulmonary veins and the LA during inspiration. Overall, as with pericardial tamponade, the transmitral velocities will be reduced in amplitude in CP. Given that the CP limits the volume of both cardiac chambers the inspiratory increase in transmitral E wave velocity will be accompanied by a simultaneous decrease in transtricuspid E wave velocity for two reasons. First, the increase in intrathoracic pressure will limit right sided filling and second, the increased filling velocity and increased amount of blood in the LV will result in a shift of the interventricular septum to the right and thus compromises RV filling during inspiration (i.e. ventricular interdependence is demonstrated). These changes in the transmitral velocities appear to be reversible with surgical treatment (pericardial stripping or pericardiectomy) of CP although this therapy has been associated with LV dilation and transient ventricular diastolic dysfunction 3,20. Much has been written about making the distinction between CP and restrictive cardiomyopathy (RCM). Both of these pathologies share the common pathophysiology of decreased left ventricular compliance, but different mechanisms account for the decreased compliance in each case. In CP the decreased compliance relates to the restrictive nature of the thickened pericardium while in RCM the restriction is related to pathology within the myocardium (e.g., infiltrative disease of the muscle or hypertrophy). Recall that severely decreased LV compliance will present a restrictive LV filling pattern (Figure 3)1-3. Figure 3 – Restrictive Diastology The differentiation of CP from RCM is best approached by assessing the patient for the described exaggerated respiratory variation in transmitral Doppler flow velocity profiles and for echocardiographic, or cardiac MRI/high resolution CT scan evidence of pericardial thickening. As previously discussed above this exaggerated respirophasic variation has been demonstrated in anesthetized patients using TEE21. Newer echocardiographic modalities that have been used to differentiate CP from RCM include color M-mode flow propagation velocities (Vp), Doppler tissue imaging (DTI) at the level of the mitral annulus, and Doppler myocardial velocity gradients (MVGs)23. DTI has been demonstrated to provide a highly sensitive and specific means to differentiate CP from RCM in one study of a homogeneous CP patient population 24,25. See Table 2. DTI has been shown to be less sensitive to preload (a limitation of transmitral pulse wave Doppler) in differentiating CP from RCM. DTI at the level of the lateral mitral annulus provides a reproducible means to align the pulse wave tissue Doppler cursor with the longitudinal axis of myocardial excursion during relaxation of the muscle. Patients with CP generally have preserved myocardial diastolic function that therefore will demonstrate normal (Em > 8 cm/sec) myocardial velocities. The converse is true for patients with RCM in that the muscle is inherently pathologic in these patients with infiltrative cardiomyopathies and thus will demonstrate abnormal (Em < 8 cm/sec). See Figure 4. Avery EG/Pericardial Disease Page 5 of 7 Figure 4. Transthoracic TDI of the lateral mitral annulus in a patient with CP. Note the high Em velocity here. Em Em Transthoracic TDI of the lateral mitral annulus in a patient with restrictive physiology. Note the diminished (Ea) or Em velocity. Rajagopalan. Am J Card 2001;87:86-94 Color M-mode, or the velocity of propagation (Vp) of transmitral flow is also recommended to be incorporated into the assessment of patients suspected of CP. Although the results of color M-mode are not as easily reproduced as DTI it is advantageous in that it appears to be preload independent and has the benefit of providing both excellent spatial and temporal resolution of diastolic mitral inflow. Color M-mode assessment of patients with CP will generally demonstrate high values of flow propagation toward the LV apex while those with RCM will have decreased V p values. See Figure 5. Figure 5. Transesophageal echocardiographic Color M-mode (Vp) of a patient with CP. Note the steep slope of the first aliasing velocity of mitral inflow (140 cm/sec)1. Transthoracic echocardiographic Color M-mode (Vp) of a patient with restrictive physiology. Note the much more gradual slope (35 cm/sec) of the first aliasing velocity as compared to the tracing displayed above. The relative sensitivities and specificities of each technique to distinguish CP from RCM are presented in Table 5. Avery EG/Pericardial Disease Page 6 of 7 Table 2. Relative Sensitivity and Specificity of Various Doppler techniques to distinguish CP from RCM. Sensitivity Specificity E wave peak (MV) (resp. variation ≥ 10%) 84 91 D wave peak (Pulmonary Vein) (resp. variation ≥ 18%) 79 91 Color M-mode Vp (slope ≥ 100 cm/s) 74 91 Tissue Doppler, (Em ≥ 8 cm/s) 89 100 Note that although these newer Doppler techniques are considered validated for use in determining diastolic dysfunction direct validation studies for TEE and patients receiving IPPV have not been performed. IV. Treatment of Constrictive Pericarditis With the less common exception of patients with transient CP (most commonly occurs in patients who have recently undergone cardiac surgical procedures) the definitive treatment is surgical pericardiectomy. In those patients who are suspected to have a transient version of the disorder conservative medical treatment and watchful waiting with the addition of systemic corticosteroids is indicated. Severely ill CP patients with multiple comorbidities may not tolerate surgical pericardiectomy and thus may not be candidates for the procedure; reported mortality in one series was 5-15%. Further, radiation induced CP is considered a relative contraindication to pericardiectomy. The medical management may involve salt restriction, diuretics and avoiding the use of beta blockers or calcium channels antagonists as affected patients develop tachycardia as this response is a helpful compensatory mechanism. If atrial fibrillation with a rapid ventricular rate develops in a CP patient then initial therapy is recommended to be digoxin and only use the beta blockers or calcium channel blockers as a last resort. Likely because it is unclear as to the exact amount of tissue that must be excised to successfully relieve the constriction not all patients will improve following pericardiectomy and while not all patients that undergo pericardiectomy will improve the reported mortality after 7 year follow up was 63% in one single center study.28 Avery EG/Pericardial Disease Page 7 of 7 References: 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. 12. 13. 14. 15. 16. 17. 18. 19. 20. 21. 22. 23. 24. 25. 26. 27. 28. Haley JH, Tajik AJ, Danielson GK, et al: Transient Constrictive Pericarditis: Causes and Natural History. J Am Coll Cardiol 2004; 43:271 Avery EG and Shernan SK. Pericardial Disease. In Comprehensive Perioperative Transesophageal Echocardiography. 2 ed. Eds Savage RM, Aronson S, Shernan SK. Lippincott, Williams and Wilkins. Chapter 39. 2010. Klick JC, Ali J, Avery EG. Echocardiographic Evaluation of Pericardial Disease. Perioperative Tranesophageal Echocardiography: A Companion to Kaplan’s Cardiac Anesthesia. Eds Reich DL, Fischer GW. Elsevier Saunders. Chapter 23. 2014. Paaladinesh T,Verhaert D, Walls MC, et al. Simultaneous Right and Left Heart Real-Time, Free Breathing CMR Flow Quantification Identifies Constrictive Physiology. J Am Coll Cardio Img 2012; 5(1): 15-24. Westenberg JJM. CMR for Assessment of Diastolic Function. 2011. Curr Cardiovasc Imaging RepI; 4(2): 149-158. Otto C. Pericardial Disease. In Textbook of Clinical Echocardiography. 3 ed. Elsevier Saunders. 2004. pp 259-275. Maisch B, Seferovic PM, Ristic AD, et al. Guidelines on the diagnosis and management of pericardial disease. Eur Heart J 2004;25:587-610. Spodick DH. Macrophysiology, microphysiology, and anatomy of the pericardium: a synopsis. Am Heart J 1992;124:1046-1051. Spodick DH. Acute Cardiac Tamponade. N Engl J Med 2003;349;684-690. Shabetai R. Pericardial and cardiac pressure. Circ 1988;77:1-5. Faehnrich JA, Noone RB, White WD, et al. Effects of positive-pressure ventilation, pericardial effusion, and cardiac tamponade on respiratory variation in transmitral flow velocities. J Cardiothorac Vasc Anesth 2003;17(1):45-50. Gatzoulis MA, Munk MD, Merchant N, et al. Isolated congenital absence of the pericardium: clinical presentation, diagnosis, and management. Ann Thorac Surg 2000;69:1209-15. Fukuda N, Oki T, Iuchi A, at al. Pulmonary and systemic venous flow patterns assessed by transesophageal Doppler echocardiography in congenital absence of the pericardium. Am J Cardiol 1995;75:1286-88. Goldschlager N, Goldman MJ. Principles of Clinical Electrocardiography. 13 th ed. Appleton and Lange. 1989. East Norwalk, CT. pp 300-302. Hoit BD, Faulx MD. Diseases of the Pericardium (Chapter 80). Fuster VR, Alexander W, O'Rourke RA, Eds. Hurst’s The Heart 11ed. The McGraw-Hill Companies, Inc. 2004. Lewinter MM, Kannani S. Pericardial Diseases (Chapter 64 – Figure 64-6B). Zipes DP, Libby P, Bonow RO, Braunwald E, Eds. In Braunwald’s Heart Disease 7 th ed. Elsevier Saunders. 2005. Burstow DJ, Oh JK, Bailey KR, et al. Cardiac tamponade: characteristic Doppler observations. Mayo Clin Pro 1989;64:312-24. Skubas NJ, Beardslee M, Barzilai B, et al. Constrictive Pericarditis: Intraoperative hemodynamic and echocardiographic evaluation of cardiac filling dynamics. Anesthesia and Analgesia 2001;92:1424-6. Oh J, Tajik J, Appleton C, et al. Preload reduction to unmask the characteristic Doppler features of constrictive pericarditi s: a new observation. Circ 1997;95(4):796799. Klein AL, Cohen GI, Pietrolungo JF, et al. Differentiation of constrictive pericarditis from restrictive cardiomyopathy by Doppler transesophageal echocardiographic measurements of respiratory variations in pulmonary vein flow. J Am Coll Cardiol 1993;22:1935-43. Abdalla IA, Murray D, Awad HE, et al. Reversal of the pattern of respiratory variation of Doppler inflow velocities in constrictive pericarditis during mechanical ventilation. J Am Soc Echocardiogr 2000;13:827-31. Senni M, Redfield M, Ling H, et al. Left ventricular systolic and diastolic function after pericardiectomy in patients with constrictive pericarditis: postoperative and serial Doppler echocardiographic findings. J Am Coll Card 1999;33:1182-8. Rodriguez, Ares MA, Vandervoot PM, et al. Does color M-mode flow propagation differentiate between patients with restrictive vs. constrictive physiology? [Abstract] J Am Coll Cardiol 1996;27:268A. Rajagopalan N, Garcia M, Rodriguez L, et al. Comparison of new Doppler echocardiographic methods to differentiate constrictive pericardial heart disease and restrictive cardiomyopathy. Am J Cardiol 2001;87:86-94. Palka P, Lange A, Donnelly E, et al. Differentiation between restrictive cardiomyopathy and constrictive pericarditis by early diastolic Doppler myocardial velocity gradient at the posterior wall. Circ 2000;102:655-62. Garcia MJ, Thomas JD, Klein AL. New Doppler echocardiographic applications for the study of diastolic function. J Amer Coll Cardiol 1998;32:865-875. Abbas AE, Appleton CP, Liu PT, et al. Congenital absence of the pericardium: case presentation and review of the literature. International J Cardiol 2005;98:21-25. Ling LH, Oh JK. Schaff HV, et al. Constrictive Pericarditis in the Modern Era: Evolving Clinical Spectrum and Impact on Outcome After Pericardiectomy. J Am Coll Cardiol 2004; 43:1445.