* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Heart Failure Due to a Post-Traumatic Calcified Pericardial Hematoma
Survey
Document related concepts
Cardiac contractility modulation wikipedia , lookup
Coronary artery disease wikipedia , lookup
Electrocardiography wikipedia , lookup
Pericardial heart valves wikipedia , lookup
Cardiothoracic surgery wikipedia , lookup
Heart failure wikipedia , lookup
Lutembacher's syndrome wikipedia , lookup
Hypertrophic cardiomyopathy wikipedia , lookup
Mitral insufficiency wikipedia , lookup
Myocardial infarction wikipedia , lookup
Echocardiography wikipedia , lookup
Quantium Medical Cardiac Output wikipedia , lookup
Dextro-Transposition of the great arteries wikipedia , lookup
Arrhythmogenic right ventricular dysplasia wikipedia , lookup
Transcript
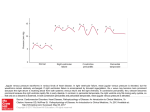
Case Reports Heart Failure Due to a Post-Traumatic Calcified Pericardial Hematoma Amit H. Manhas, MD Robert T. Martin, MD, FACC George J. Reul, MD Raymond F. Stainback, MD Chest-wall trauma can produce bleeding into the pericardium and initiate a process of inflammation, calcification, and scarring that may eventually produce pericardial constriction. Herein, we present an unusual case of a man who experienced chest trauma at age 16 years, and developed heart failure 40 years later secondary to a large, calcified pericardial hematoma. During its prolonged genesis, the pericardial mass became deeply embedded in the myocardium and produced evidence of both constrictive and restrictive cardiomyopathy. Despite attempted surgical resection, the lesion could not be completely removed, nor could its hemodynamic impact be completely resolved. (Tex Heart Inst J 2008;35(3): 345-8) Key words: Calcinosis/ complications/etiology; cardiomyopathy, restrictive/ physiopathology/ultrasonography; echocardiography, Doppler, pulsed; heart injuries/complications/ pathology; hematoma/ complications/etiology; pericarditis, constrictive/ diagnosis/etiology/physiopathology/radiography; pericardium/physiology; thoracic injuries/complications; time factors From: Section of Cardiology (Dr. Manhas), Baylor College of Medicine, Houston, Texas 77030; Ark-La-Tex Cardiology, APMC (Dr. Martin), Shreveport, Louisiana 71135; and departments of Cardiovascular Surgery (Dr. Reul) and Adult Cardiology (Dr. Stainback), Texas Heart Institute at St. Luke’s Episcopal Hospital, Houston, Texas 77030 Address for reprints: Raymond F. Stainback, MD, 6624 Fannin, Suite 2480, Houston, TX 77030 E-mail: [email protected] © 2008 by the Texas Heart ® Institute, Houston Texas Heart Institute Journal oth constrictive pericarditis and restrictive cardiomyopathy can produce heart failure, often with a preserved left ventricular ejection fraction. In some cases, constrictive and restrictive physiology may coexist. Differentiating these 2 heart failure mechanisms, although difficult, is important, because surgical intervention can cure constrictive pericardial disease. Therefore, numerous clinical, radiologic, and echocardiographic criteria have been defined to help distinguish between these conditions.1 In some cases, however, as in constrictive–restrictive cardiomyopathy, classification and treatment planning may be particularly difficult. Herein, we describe the case of a patient who survived severe chest trauma at age 16 years and developed symptoms of congestive heart failure 40 years later. Case Report A 58-year-old man was admitted to the hospital with progressive shortness of breath, edema, and anorexia. Over 2 years, he had gradually developed symptoms of New York Heart Association (NYHA) functional class III–IV congestive heart failure. At age 16 years, he had incurred chest-wall trauma upon being thrown into a dashboard during an automobile accident. Immediately after that accident, he had required no medical intervention. Later that year, however, he developed volume overload and failure to thrive. Physical examination revealed massive hepatomegaly, peripheral edema, and a large right-sided pleural effusion. After a steroid regimen proved unsuccessful, pleural decortication and a pericardiectomy were performed. The right lung was atelectatic and rubbery. During pericardial stripping, the moderately thickened, heavily calcified pericardium adhered to the myocardium. Calcium plaques were embedded in the myocardium and could not be completely removed. After surgery, the patient gradually recovered and had a normal life. After 40 years of good health, including vigorous exercise, the patient began to show symptoms of heart failure. He complained of decreasing exercise tolerance, progressive lower-extremity edema, a persistent dry cough, and decreased appetite. He did not respond to diuretic or asthma medications prescribed by his local physicians. Noninvasive imaging, performed by his cardiologists, showed a large pericardial mass and possible constrictive pericarditis. When the patient was referred to our institution, he was in NYHA functional class III–IV and was no longer able to work. Physical examination revealed a thin man who appeared chronically ill and fatigued. He had a blood pressure of 115/70 mmHg (pulsus paradoxus, 5–10 mmHg), a heart rate of 98 beats/min, and unlabored respirations at 18 per minute. His jugular venous pressure was elevated to 6 cm at 30 degrees with a prominent A wave. The apical impulse was visible and easily palpable at the left nipple. The S1 and S2 sounds were Calcified Pericardial Hematoma 345 normal, without physiologic splitting; there was no precordial knock and no S3 or S4 sound. The right side of his thorax was slightly smaller than the left. His lungs were clear to auscultation, and excursion was decreased on the right side. His abdomen was soft with a slight fluid wave. The liver span was increased, with obvious hepatojugular reflux, and the spleen tip was palpable. The lower extremities had 3+ pitting edema bilaterally to the knees. Echocardiography (Figs. 1A and 1B) showed a large intrapericardial mass centered in the left atrioventricular groove, mild biatrial enlargement, a small left ventricle with an end-diastolic diameter of 2.4 cm, and normal overall systolic function (left ventricular ejection fraction, 0.50). The intrapericardial mass produced a notable systolic and diastolic deformity of the adjacent ventricle, and a pronounced ventricular diastolic septal bounce was noted. The mitral inflow pattern, illustrated by pulsed-wave Doppler methods, was restrictive (Fig. 1C) and showed no significant respiratory variation (Fig. 1D). Cardiac magnetic resonance imaging (MRI) (Fig. 2) confirmed the presence of a large (14 × 10 × 9-cm), complex, crescent-shaped intrapericardial mass inferolateral to the left ventricle. The mass appeared to be extensively calcified, with both soft-tissue and proteinaceous-fluid components. Left- and rightsided heart catheterization showed normal coronary arteries and only minimal ventricular discordance of peak systolic pressures during respiration (Fig. 3). Moderate pulmonary hypertension was accompanied by a severely elevated right atrial pressure and equalized diastolic pressures (in mmHg): right atrial, 19; right ventricular, 46/19; pulmonary artery, 47/18; pulmonary capillary wedge, 22; left ventricular systolic, 103; and left ventricular end-diastolic, 22. The extensive, complex, partially calcified intrapericardial mass invaded the adjacent epicardium, producing a mixed constrictive–restrictive physiology. Although we thought that restrictive cardiomyopathy was playing a substantial role in the patient’s condition, we believed that excision of the large mass might lead to palliation by relieving the pericardial constriction. A B C D Fig. 1 A) Apical 4-chamber echocardiographic view shows the pericardial mass deforming and restricting the left ventricle. B) Parasternal long-axis echocardiographic view shows the pericardial mass impinging on the posterior lateral wall of the left ventricle. C) Pulsedwave Doppler studies suggest a severely restrictive filling pattern, due to the early-to-late diastolic filling ratio of >2 and the reduced deceleration time. D) Pulsed-wave Doppler studies failed to show the respiratory variation in flow across the mitral valve that is typically seen in constrictive pericarditis. Real-time motion are available at texasheart.org/journal. Click here forimages real-time motion image: Fig. 1A. 346 Calcified Pericardial Hematoma Click here for real-time motion image: Fig. 1B. Volume 35, Number 3, 2008 Fig. 2 Cardiac magnetic resonance imaging reveals a complex retrocardiac mass with components of fluid and soft-tissue density. Real-time image ismotion available at texasheart.org/journal. Click heremotion for real-time image: Fig. 2. In the operating room, the mass appeared to be an old calcified hematoma that was adhering to the myocardium (Figs. 4A and 4B). After resecting the mass, we attempted to dissect the adherent debris and calcification from the epicardium. Much of the calcium was deeply embedded in the muscle, however, and could not be completely excised. Pathologic examination of the mass revealed benign soft tissue with xanthogranulomatous inflammation, sclerosis, and associated old hemorrhage and focal calcification. After surgery, the patient continued to experience volume overload and minimally improved cardiac hemodynamics. Repeat echocardiography and MRI revealed a persistent hollow shell or potential space in the area of the previous mass. Repeat echocardiography of the heart continued to show a prominent diastolic deformity where the mass had been located. The patient experienced persistent respiratory insufficiency and, after a 3-month hospitalization, died of multiorgan failure related to low cardiac output. Discussion Fig. 3 Pressure-tracing from the right and left ventricles shows a slight discordance with respiration. This pattern is typically seen in constrictive pericarditis, because the diminished pulmonary capillary wedge pressure-to-left atrial pressure gradient results in preferential right-sided filling during inspiration. A Chest trauma frequently causes bleeding into the pericardium. Researchers have found that blood injected into the pericardium of experimental animals generally resolves without consequences. After trauma, however, the fibrinolytic and absorptive functions of the pericardium are impaired, and adhesions may form between the pericardial layers.2 Fibrosis and calcification of the pericardium may produce constrictive pericarditis, which is a well-known complication of blunt chest trauma.3,4 Our patient had a large, organized pericardial hematoma due to such trauma. Although he underwent partial pericardial stripping 1 year after his initial injury, the disease process was not arrested. This may be because he already had significant epicardial involvement, and complete resection of the diseased tissue could not be achieved. Given the duration of the disease process B Fig. 4 A) At surgery, the pericardium was found to be heavily calcified and closely adherent to the myocardium. B) Large, calcified debris was removed from the pericardial space. Texas Heart Institute Journal Calcified Pericardial Hematoma 347 and the extent of epicardial involvement, he probably experienced a self-perpetuating cycle of injury, bleeding, and fibrocalcific reaction. Post-traumatic formation of a large, organized, calcified hematoma is rare, but a few such lesions have been described in the medical literature.5-11 In those patients, the time from the initial injury to the clinical manifestation of constriction was sometimes prolonged. In a literature review presented by Brown and Ivey,5 the time from injury to surgery ranged from 3 to 20 years. Remarkably, our patient had a 40-year period of clinical stability before his health deteriorated. This long latent period highlights the persistent, insidious nature of the disease, which had progressed considerably before it produced any clinical warning signs. Our patient had a complex lesion with characteristics of both constrictive and restrictive disease.12,13 The constrictive elements were equalization of diastolic pressures in all chambers, a pronounced septal bounce, and a slight discordance of ventricular pressures during respiration. Restrictive elements included biatrial enlargement, elevated systolic pulmonary artery pressure, a restrictive mitral inflow pattern, and minimal respiratory variation in mitral inflow. These findings portended the combined pericardial–myocardial nature of this lesion, which was verified during surgery. Although the bulk of the offending mass was removed, almost no improvement was seen in the patient’s hemodynamic performance. Therefore, patients with findings of both constrictive and restrictive disease during the preoperative evaluation of pericardial-mass lesions may have an incomplete clinical response to surgical resection. In such patients, the prognosis seems especially grim, and treatment options may be limited to palliation or cardiac transplantation. 348 Calcified Pericardial Hematoma References 1. Asher CR, Klein AL. Diastolic heart failure: restrictive cardiomyopathy, constrictive pericarditis, and cardiac tamponade: clinical and echocardiographic evaluation. Cardiol Rev 2002;10(4):218-29. 2. Sbokos CG, Karayannacos PE, Kontaxis A, Kambylafkas J, Skalkeas GD. Traumatic hemopericardium and chronic constrictive pericarditis. Ann Thorac Surg 1977;23(3):225-9. 3. Goldstein S, Yu PN. Constrictive pericarditis after blunt chest trauma. Am Heart J 1965;69:544-50. 4. Gothman B, Johansson L, Silander T. Post-traumatic constrictive pericarditis. Acta Chir Scand 1963;125:77-80. 5. Brown DL, Ivey TD. Giant organized pericardial hematoma producing constrictive pericarditis: a case report and review of the literature. J Trauma 1996;41(3):558-60. 6. Campbell PG, De Belder MA, Hartley R. Pericardial constriction secondary to calcified intrapericardial haematoma. Heart 2001;86(2):160. 7. Dunlap TE, Sorkin RP, Mori KW, Popat KD. Massive organized intrapericardial hematoma mimicking constrictive pericarditis. Am Heart J 1982;104(6):1373-5. 8. Hartl WH, Kreuzer E, Reuschel-Janetschek E, Schmidt D, Reichart B. Pericardial mass mimicking constrictive pericarditis. Ann Thorac Surg 1991;52(3):557-9. 9. Isaacs D, Stark P, Nichols C, Antevil J, Shabetai R. Post-traumatic pericardial calcification. J Thorac Imaging 2003;18(4): 250-3. 10. Leslie SJ, Brackenbury E, Spratt JC. Calcified mediastinal haematoma: a rare case of cardiac constriction. Heart 2004; 90(8):846. 11. Taylor MW, Garber JC, Boswell WC, Boyd CR. Delayed hemopericardium and associated pericardial mass after blunt chest trauma. Am Surg 2003;69(4):343-5. 12. Goldstein JA. Cardiac tamponade, constrictive pericarditis, and restrictive cardiomyopathy. Curr Probl Cardiol 2004;29 (9):503-67. 13. Hatle LK, Appleton CP, Popp RL. Differentiation of constrictive pericarditis and restrictive cardiomyopathy by Doppler echocardiography. Circulation 1989;79(2):357-70. Volume 35, Number 3, 2008