* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Edi Schwager
Serotonin syndrome wikipedia , lookup
Drug design wikipedia , lookup
Polysubstance dependence wikipedia , lookup
Pharmacokinetics wikipedia , lookup
Drug discovery wikipedia , lookup
Pharmacogenomics wikipedia , lookup
Pharmacognosy wikipedia , lookup
Prescription drug prices in the United States wikipedia , lookup
Pharmaceutical industry wikipedia , lookup
Prescription costs wikipedia , lookup
Drug interaction wikipedia , lookup
Amphetamine wikipedia , lookup
Neuropharmacology wikipedia , lookup
Drugs
Edi Schwager
PDF generated using the open source mwlib toolkit. See http://code.pediapress.com/ for more information.
PDF generated at: Sun, 22 May 2011 16:29:59 UTC
Contents
Articles
Adenosine reuptake inhibitor
1
Amphetamine
4
Ataractive
18
Cathinone
19
Dextrorphan
21
Dopamine reuptake inhibitor
23
FX-1006A
28
GABA agonist
28
Melatonin
29
Mephedrone
45
Norepinephrine releasing agent
63
Norepinephrine reuptake inhibitor
64
Propylhexedrine
69
Psychoanaleptic
73
Selective serotonin releasing agent
73
Serotonin releasing agent
74
Serotonin reuptake inhibitor
78
References
Article Sources and Contributors
83
Image Sources, Licenses and Contributors
85
Article Licenses
License
86
Adenosine reuptake inhibitor
1
Adenosine reuptake inhibitor
An adenosine reuptake inhibitor (AdoRI) is a type of drug
which acts as a reuptake inhibitor for the purine nucleoside and
neurotransmitter adenosine by blocking the action of one or more
of the equilibrative nucleoside transporters (ENTs).[1] [2] [3] This in
turn leads to increased extracellular concentrations of adenosine
and therefore an increase in adenosinergic neurotransmission.
List of AdoRIs
• Acadesine[4]
• Acetate[4]
• Barbiturates[5] [6]
• Benzodiazepines[5] [7] [8] [9] [10] [11] [12]
• Calcium Channel Blockers[4]
•
•
•
•
•
•
•
•
•
•
•
•
•
•
•
•
•
•
•
•
•
•
•
•
•
•
•
•
Carbamazepine[4]
Carisoprodol[13] [14] [15]
Cilostazol[16]
Cyclobenzaprine[17] [18]
Dilazep[1]
Dipyridamole[1] [19]
Estradiol[20]
Ethanol (Alcohol)[21]
Flumazenil[4]
Hexobendine[22]
Hydroxyzine[23]
Indomethacin[4]
Inosine[24]
KF24345[25]
Meprobamate[13] [14]
Nitrobenzylthioguanosine[26]
Nitrobenzylthioinosine[1] [27]
Papaverine[28]
Pentoxifylline[29]
Phenothiazines[17] [30]
Phenytoin[31]
Progesterone[20]
Propentofylline[32]
Propofol[5] [33]
Puromycin[17]
R75231[34]
RE 102 BS[35]
Soluflazine[36] [37]
• Toyocamycin[17]
• Tracazolate[23]
Adenosine
Adenosine reuptake inhibitor
• Tricyclic Antidepressants[17] [18]
References
[1] SenGupta DJ, Unadkat JD. (2004). "Glycine 154 of the equilibrative nucleoside transporter, hENT1, is important for nucleoside transport and
for conferring sensitivity to the inhibitors nitrobenzylthioinosine, dipyridamole, and dilazep.". Biochem Pharmacol. 67 (3): 453–458.
doi:10.1016/j.bcp.2003.09.018. PMID 15037197.
[2] Endres CJ, Sengupta DJ, Unadkat JD. (2004). "Mutation of leucine-92 selectively reduces the apparent affinity of inosine, guanosine,
NBMPR [S6-(4-nitrobenzyl)-mercaptopurine riboside] and dilazep for the human equilibrative nucleoside transporter, hENT1.". Biochem J.
380 (1): 131–137. doi:10.1042/BJ20031880. PMC 1224139. PMID 14759222.
[3] Chaudary N, Naydenova Z, Shuralyova I, Coe IR. (2004). "The adenosine transporter, mENT1, is a target for adenosine receptor signaling
and protein kinase Cepsilon in hypoxic and pharmacological preconditioning in the mouse cardiomyocyte cell line, HL-1.". J Pharmacol Exp
Ther. 310 (3): 1190–1198. doi:10.1124/jpet.104.067157. PMID 15131243.
[4] Goldfrank, Lewis R.; Neal Flomenbaum, Mary Ann Howland, Robert S. (2006). Goldfrank's toxicologic emergencies. p. 243.
[5] Narimatsu E, Niiya T, Kawamata M, Namiki A. (2006). "[The mechanisms of depression by benzodiazepines, barbiturates and propofol of
excitatory synaptic transmissions mediated by adenosine neuromodulation]". Masui. 55 (6): 684–691. PMID 16780077.
[6] Tohdoh Y, Narimatsu E, Kawamata M, Namiki A. (2000). "The involvement of adenosine neuromodulation in pentobarbital-induced field
excitatory postsynaptic potentials depression in rat hippocampal slices.". Anesth Analg. 91 (6): 1537–1541.
doi:10.1097/00000539-200012000-00044. PMID 11094014.
[7] Patel J, Marangos PJ, Skolnick P, Paul SM, Martino AM. (1982). "Benzodiazepines are weak inhibitors of [3H]nitrobenzylthioinosine
binding to adenosine uptake sites in brain.". Neurosci Lett. 29 (1): 79–82. doi:10.1016/0304-3940(82)90368-8. PMID 7070715.
[8] York MJ, Davies LP. (1982). "The effect of diazepam on adenosine uptake and adenosine-stimulated adenylate cyclase in guinea-pig brain.".
Can J Physiol Pharmacol. 60 (3): 302–307. PMID 6280826.
[9] Ujfalusi A, Cseppentö A, Nagy E, Szabó JZ, Kovács P, Szentmiklósi AJ. (1999). "Sensitization by chronic diazepam treatment of A2A
adenosine receptor-mediated relaxation in rat pulmonary artery.". Life Sci. 64 (2): PL19–25. doi:10.1016/S0024-3205(98)00547-5.
PMID 10069495.
[10] Narimatsu E, Niiya T, Kawamata M, Namiki A. (2008). "Adenosine and adenosine uptake inhibitors potentiate the neuromuscular blocking
action of rocuronium mediated by adenosine A(1) receptors in isolated rat diaphragms.". Acta Anaesthesiol Scand. 52 (10): 1415–1422.
doi:10.1111/j.1399-6576.2008.01714.x. PMID 19025536.
[11] Bender AS, Hertz L. (1986). "Similarities of adenosine uptake systems in astrocytes and neurons in primary cultures.". Neurochem Res. 11
(11): 1507–1524. doi:10.1007/BF00965770. PMID 2891057.
[12] O'Regan MH, Phillis JW. (1988). "Potentiation of adenosine-evoked depression of rat cerebral cortical neurons by triazolam.". Brain Res.
445 (2): 376–379. doi:10.1016/0006-8993(88)91203-6. PMID 3370471.
[13] Phillis JW, Delong RE. (1984). "A purinergic component in the central actions of meprobamate.". Eur J Pharmacol. 101 (3-4): 295–297.
doi:10.1016/0014-2999(84)90174-2. PMID 6468504.
[14] DeLong RE, Phillis JW, Barraco RA. (1985). "A possible role of endogenous adenosine in the sedative action of meprobamate.". Eur J
Pharmacol. 118 (3): 359–362. doi:10.1016/0014-2999(85)90149-9. PMID 4085561.
[15] Gonzalez LA, Gatch MB, Taylor CM, Bell-Horner CL, Forster MJ, Dillon GH. (2009). "[Carisoprodol-mediated modulation of GABAA
receptors: in vitro and in vivo studies.". J Pharmacol Exp Ther. 329 (2): 827–837. doi:10.1124/jpet.109.151142. PMC 2672873.
PMID 19244096.
[16] Wang S, Cone J, Fong M, Yoshitake M, Kambayashi Ji, Liu Y. (2001). "Interplay between inhibition of adenosine uptake and
phosphodiesterase type 3 on cardiac function by cilostazol, an agent to treat intermittent claudication.". J Cardiovasc Pharmacol. 38 (5):
775–783. doi:10.1097/00005344-200111000-00014. PMID 11602824.
[17] Phillis JW, Wu PH. (1982). "The effect of various centrally active drugs on adenosine uptake by the central nervous system.". Comp
Biochem Physiol C. 72 (2): 179–187. doi:10.1016/0306-4492(82)90082-X. PMID 6128137.
[18] Phillis JW. (1984). "Potentiation of the action of adenosine on cerebral cortical neurones by the tricyclic antidepressants.". Br J Pharmacol.
83 (2): 567–575. PMC 1987110. PMID 6487906.
[19] Stein MB, Black B, Brown TM, Uhde TW. (1993). "Lack of efficacy of the adenosine reuptake inhibitor dipyridamole in the treatment of
anxiety disorders.". Biol Psychiatry. 33 (8-9): 647–650. doi:10.1016/0006-3223(93)90105-M. PMID 8329495.
[20] Phillis JW, Bender AS, Marszalec W. (1985). "Estradiol and progesterone potentiate adenosine's depressant action on rat cerebral cortical
neurons.". Gen Pharmacol 16 (6): 609–612. PMID 2935451.
[21] Allen-Gipson DS, Jarrell JC, Bailey KL, Robinson JE, Kharbanda KK, Sisson JH, Wyatt TA. (2009). "Ethanol Blocks Adenosine Uptake
via Inhibiting the Nucleoside Transport System in Bronchial Epithelial Cells.". Alcohol Clin Exp Res. 33 (5): 791–8.
doi:10.1111/j.1530-0277.2009.00897.x. PMC 2940831. PMID 19298329.
[22] Verma A, Houston M, Marangos PJ. (1985). "Solubilization of an adenosine uptake site in brain.". J Neurochem. 45 (2): 596–603.
doi:10.1111/j.1471-4159.1985.tb04028.x. PMID 2989430.
[23] Phillis JW, Wu PH, Coffin VL. (1983). "Inhibition of adenosine uptake into rat brain synaptosomes by prostaglandins, benzodiazepines and
other centrally active compounds.". Gen Pharmacol. 14 (5): 475–479. PMID 6416920.
2
Adenosine reuptake inhibitor
[24] Ngai AC, Monsen MR, Ibayashi S, Ko KR, Winn HR. (1989). "Effect of inosine on pial arterioles: potentiation of adenosine-induced
vasodilation.". Am J Physiol. 256 (3 (Pt2)): H603–H606. PMID 2923227.
[25] Noji T, Nan-ya K, Mizutani M, Katagiri C, Sano J, Takada C, Nishikawa S, Karasawa A, Kusaka H. (2002). "KF24345, an adenosine uptake
inhibitor, ameliorates the severity and mortality of lethal acute pancreatitis via endogenous adenosine in mice.". Eur J Pharmacol. 454 (1):
85–93. doi:10.1016/S0014-2999(02)02476-7. PMID 12409009.
[26] Lee CM, Cheung WT. (1985). "Inhibitory effect of adenosine on electrically evoked contractions in the rat vas deferens: pharmacological
characterization.". Neurosci Lett. 59 (1): 47–52. doi:10.1016/0304-3940(85)90213-7. PMID 2995881.
[27] Marangos PJ, Patel J, Clark-Rosenberg R, Martino AM. (1982). "[3H]nitrobenzylthioinosine binding as a probe for the study of adenosine
uptake sites in brain.". J Neurochem. 39 (1): 184–191. doi:10.1111/j.1471-4159.1982.tb04717.x. PMID 7086410.
[28] Coffin VL, Taylor JA, Phillis JW, Altman HJ, Barraco RA. (1984). "Behavioral interaction of adenosine and methylxanthines on central
purinergic systems.". Neurosci Lett. 47 (2): 91–98. doi:10.1016/0304-3940(84)90412-9. PMID 6205333.
[29] Shi D, Daly JW. (1999). "Chronic effects of xanthines on levels of central receptors in mice.". Cell Mol Neurobiol. 19 (6): 719–732.
doi:10.1023/A:1006901005925. PMID 10456233.
[30] Phillis JW. (1985). "Chlorpromazine and trifluoperazine potentiate the action of adenosine on rat cerebral cortical neurons.". Gen
Pharmacol. 16 (1): 19–24. PMID 2984085.
[31] Phillis JW. (1984). "Interactions of the anticonvulsants diphenylhydantoin and carbamazepine with adenosine on cerebral cortical neurons.".
Epilepsia. 25 (6): 765–772. doi:10.1111/j.1528-1157.1984.tb03489.x. PMID 6510384.
[32] Andiné P, Rudolphi KA, Fredholm BB, Hagberg H. (1990). "Effect of propentofylline (HWA 285) on extracellular purines and excitatory
amino acids in CA1 of rat hippocampus during transient ischaemia.". Br J Pharmacol. 100 (4): 814–818. PMC 1917600. PMID 2207501.
[33] Ohmori H, Sato Y, Namiki A. (2004). "The anticonvulsant action of propofol on epileptiform activity in rat hippocampal slices.". Anesth
Analg. 99 (4): 1095–1101. doi:10.1213/01.ANE.0000130356.22414.2B. PMID 15385357.
[34] Noji T, Nan-ya K, Katagiri C, Mizutani M, Sano J, Nishikawa S, Karasawa A, Kusaka H. (2002). "Adenosine uptake inhibition ameliorates
cerulein-induced acute pancreatitis in mice.". Pancreas. 25 (4): 387–392. doi:10.1097/00006676-200211000-00011. PMID 12409834.
[35] Gresele P, Arnout J, Deckmyn H, Vermylen J. (1986). "Mechanism of the antiplatelet action of dipyridamole in whole blood: modulation of
adenosine concentration and activity.". Thromb Haemost. 55 (1): 12–18. PMID 3704998.
[36] Bauman LA, Mahle CD, Boissard CG, Gribkoff VK. (1992). "Age-dependence of effects of A1 adenosine receptor antagonism in rat
hippocampal slices.". J Neurophysiol. 68 (2): 629–638. PMID 1388201.
[37] Boissard CG, Gribkoff VK. (1993). "The effects of the adenosine reuptake inhibitor soluflazine on synaptic potentials and population
hypoxic depolarizations in area CA1 of rat hippocampus in vitro.". Neuropharmacology. 32 (2): 149–155.
doi:10.1016/0028-3908(93)90095-K. PMID 8383814.
3
Amphetamine
4
Amphetamine
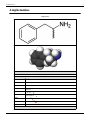
Amphetamine
Systematic (IUPAC) name
(±)-1-phenylpropan-2-amine
Identifiers
[1]
[2]
CAS number
300-62-9
ATC code
N06 BA01
PubChem
CID 3007
DrugBank
DB00182
ChemSpider
13852819
UNII
CK833KGX7E
KEGG
D07445
ChEMBL
CHEMBL405
Synonyms
alpha-methylbenzeneethanamine, alpha-methylphenethylamine, beta-phenyl-isopropylamine
405-41-4
[3]
[4]
[5]
[6]
[7]
[8]
[9]
Chemical data
Amphetamine
5
Formula
C9H13N
Mol. mass
135.2084
SMILES
eMolecules
[10]
& PubChem
[11]
Physical data
Melt. point
285–281 °C (545–538 °F)
Solubility in water 50–100 mg/mL (16C°) mg/mL (20 °C)
Pharmacokinetic data
Bioavailability
nasal 75%; rectal 95–99%; intravenous 100%
Protein binding
15–40%
Metabolism
Hepatic (CYP2D6)
Half-life
12h average for d-isomer, 13h for l-isomer
Excretion
Renal; significant portion unaltered
[12]
Therapeutic considerations
Pregnancy cat.
C(US)
Legal status
Controlled (S8) (AU) Schedule II (CA) ? (UK) Schedule II (US) ℞ Prescription only
Routes
Oral, Intravenous, Vaporization, Insufflation, Rectal, Sublingual
(what is this?) (verify)
[13]
Amphetamine (USAN) or amfetamine (INN) is a psychostimulant drug of the phenethylamine class which
produces increased wakefulness and focus in association with decreased fatigue and appetite.
Brand names of medications that contain, or metabolize into, amphetamine include Adderall, Dexedrine, Dextrostat,
Desoxyn,[14] ProCentra, and Vyvanse, as well as Benzedrine in the past.
The drug is also used recreationally and as a performance enhancer. Recreational users of amphetamine have coined
numerous street names for amphetamine, such as "speed". The European Monitoring Centre for Drugs and Drug
Addiction reports the typical retail price of diluted amphetamine in Europe varied between €3 and €15 ($4 to $21.55
USD) a gram in half of the reporting countries. Street amphetamine is typically about 10% pure.[15] The name
amphetamine is derived from its chemical name alpha-methyl phenethylamine.
Amphetamine
6
Effects
Physical effects
Physical effects of dextroamphetamine can include
hyperactivity, dilated pupils, blood shot eyes, flushing,
restlessness, dry mouth, bruxism, headache,
tachycardia, bradycardia, tachypnea, hypertension,
hypotension, fever, diaphoresis, diarrhea, constipation,
blurred vision, aphasia, dizziness, twitching, insomnia,
numbness, palpitations, arrhythmias, tremors, dry
and/or itchy skin, acne, pallor, convulsions, and with
chronic and/or high doses, seizure, stroke, coma, heart
attack and death can occur.[17] [18] [19] [20] [21] There is
also significant research which highlights the possible
neurotoxic effects of amphetamine on the dopaminergic
system, even in clinical doses.[22]
Psychological effects
Data from The Lancet suggests amphetamine is ranked the 8th most
[16]
addictive and 6th most harmful of 20 popular recreational drugs.
Psychological effects can include euphoria, anxiety,
increased libido, alertness, concentration, energy, self-esteem, self-confidence, sociability, irritability, aggression,
psychosomatic disorders, psychomotor agitation, grandiosity, excessive feelings of power and superiority, repetitive
and obsessive behaviors, paranoia, and with chronic and/or high doses, amphetamine psychosis can occur.[23] [24]
Withdrawal effects
Withdrawal symptoms of amphetamine primarily consist of mental fatigue, mental depression and increased
appetite. Symptoms may last for days with occasional use and weeks or months with chronic use, with severity
dependent on the length of time and the amount of amphetamine used. Withdrawal symptoms may also include
anxiety, agitation, excessive sleep, vivid or lucid dreams, deep REM sleep and suicidal ideation.[25] [26] [27]
Side effects
Contraindications
Amphetamine elevates cardiac output and blood pressure making it dangerous for use by patients with a history of
heart disease or hypertension. Amphetamine can cause a life-threatening complication in patients taking MAOI
antidepressants. The use of amphetamine and amphetamine-like drugs is contraindicated in patients with
narrow-angle glaucoma or anatomically narrow angles. Like other sympathomimetic amines, amphetamine can
induce transient mydriasis. In patients with narrow angles, pupillary dilation can provoke an acute attack of
angle-closure glaucoma. These agents should also be avoided in patients with other forms of glaucoma, as mydriasis
may occasionally increase intraocular pressure.[28]
Amphetamine has been shown to pass through into breast milk. Because of this, mothers taking amphetamine are
advised to avoid breastfeeding during their course of treatment.[29]
Amphetamine
Dependence and addiction
Tolerance is developed rapidly in amphetamine abuse; therefore, periods of extended use require increasing amounts
of the drug in order to achieve the same effect.[30]
Overdose
An amphetamine overdose is rarely fatal but can lead to a number of different symptoms, including psychosis, chest
pain, and hypertension.
Psychosis
Abuse of amphetamines can result in a stimulant psychosis that can present as a number of psychotic disorders (i.e.
paranoia, hallucinations, delusions). The intensity and duration of symptoms may vary, but unlike true psychotic
disorders (i.e. schizophrenia), stimulant psychoses are not considered to be permanent and will eventually resolve
upon discontinuation of the drug's use.
Mechanism of action
Primary sites of action
Amphetamine exerts its behavioral effects by modulating several key neurotransmitters in the brain, including
dopamine, serotonin, and norepinephrine. However, the activity of amphetamine throughout the brain appears to be
specific;[31] certain receptors that respond to amphetamine in some regions of the brain tend not to do so in other
regions. For instance, dopamine D2 receptors in the hippocampus, a region of the brain associated with forming new
memories, appear to be unaffected by the presence of amphetamine.[31]
The major neural systems affected by amphetamine are largely implicated in the brain’s reward circuitry. Moreover,
neurotransmitters involved in various reward pathways of the brain appear to be the primary targets of
amphetamine.[32] One such neurotransmitter is dopamine, a chemical messenger heavily active in the mesolimbic
and mesocortical reward pathways. Not surprisingly, the anatomical components of these pathways—including the
striatum, the nucleus accumbens, and the ventral striatum—have been found to be primary sites of amphetamine
action.[33] [34]
The fact that amphetamine influences neurotransmitter activity specifically in regions implicated in reward provides
insight into the behavioral consequences of the drug, such as the stereotyped onset of euphoria.[34] A better
understanding of the specific mechanisms by which amphetamine operates may increase our ability to treat
amphetamine addiction, as the brain’s reward circuitry has been widely implicated in addictions of many types.[35]
Endogenous amphetamines
Amphetamine has been found to have several endogenous analogues; that is, molecules of a similar structure found
naturally in the brain.[36] l-Phenylalanine and β-Phenethylamine are two examples, which are formed in the
peripheral nervous system as well as in the brain itself. These molecules are thought to modulate levels of excitement
and alertness, among other related affective states.
Dopamine
The most widely studied neurotransmitter with regard to amphetamine action in the central nervous system is
dopamine. All of the addictive drugs appear to enhance dopamine neurotransmission, including amphetamine and
methamphetamine.[37] Studies have shown that in select regions, amphetamine increases the concentrations of
dopamine in the synaptic cleft, thereby heightening the response of the post-synaptic neuron.[38] This specific action
hints at the hedonic response to the drug as well as to the drug’s addictive quality.
7
Amphetamine
The specific mechanisms by which amphetamine affects dopamine concentrations have been studied extensively.
Currently, two major hypotheses have been proposed, which are not mutually exclusive. One theory emphasizes
amphetamine’s actions on the vesicular level, increasing concentrations of dopamine in the cytosol of the
pre-synaptic neuron.[36] [39] The other focuses on the role of the dopamine transporter DAT, and proposes that
amphetamine may interact with DAT to induce reverse transport of dopamine from the presynaptic neuron into the
synaptic cleft.[32] [40] [41] [42]
The former hypothesis is backed by studies from David Sulzer's lab at Columbia University demonstrating that
injections of amphetamine result in rapid increases of cytosolic dopamine concentrations, while the drug decreases
the number of dopamine molecules inside the synaptic vesicle.[43] [44] Amphetamine is a substrate for a specific
neuronal synaptic vesicle uptake transporter called VMAT2. When amphetamine is taken up by VMAT2, the vesicle
releases dopamine molecules into the cytosol in exchange. The redistributed dopamine is then believed to interact
with DAT to promote reverse transport.[36] Amphetamine and amphetamine derivatives are also weak bases that
accept protons, and can collapse acidic pH gradients in the vesicles that would otherwise provide free energy for
neurotransmitter accumulation: the "weak base hypothesis" of amphetamine action suggests that collapse of this free
energy contributes to redistribution of dopamine from very high (molar) concentrations in the vesicles to the
cytosol.[37] [45] Calcium may be a key molecule involved in the interactions between amphetamine and VMATs.[39]
The increase of cytosolic dopamine appears to trigger neurotoxicity, as dopamine readily auto-oxidizes, so that
amphetamine or methamphetamine's increase in cytosolic dopamine can lead to oxidative stress in the cytosol that in
turn promotes autophagy-related degradation of dopamine axons and dendrites.[46]
The second hypothesis of amphetamine action on the plasma membrane dopamine transporter postulates a direct
interaction between amphetamine and the DAT. The activity of DAT is believed to depend on specific
phosphorylating kinases, such as protein kinase c, specifically PKC-β.[42] Upon phosphorylation, DAT undergoes a
conformational change that results in the transportation of DAT-bound dopamine from the extracellular to the
intracellular environment.[41] In the presence of amphetamine, however, DAT has been observed to function in
reverse, spitting dopamine out of the presynaptic neuron and into the synaptic cleft.[40] Thus, beyond inhibiting
reuptake of dopamine, amphetamine also stimulates the release of dopamine molecules into the synapse.[32]
In support of the above hypothesis, it has been found that PKC-β inhibitors eliminate the effects of amphetamine on
extracellular dopamine concentrations in the striatum of rats.[42] This data suggests that the PKC-β kinase may
represent a key point of interaction between amphetamine and the DAT transporter.
Additional actions of amphetamine contribute to it's ability to release dopamine from neurons, including action as an
inhibitor of monoamine oxidase, an enzyme responsible for dopamine breakdown in the cytosol; an ability to
enhance dopamine synthesis apparently via actions on the enzyme tyrosine hydroxylase, which synthesizes the
dopamine precursor L-DOPA; and some blockade of the DAT, an action that amphetamine shares with cocaine.[47]
Due to the combination of these actions, amphetamine can release far more dopamine that can cocaine or other
addictive drugs.[37] [48]
Serotonin
Amphetamine has been found to exert similar effects on serotonin as on dopamine.[49] Like DAT, the serotonin
transporter SERT can be induced to operate in reverse upon stimulation by amphetamine.[50] This mechanism is
thought to rely on the actions of calcium ions, as well as on the proximity of certain transporter proteins.[50]
The interaction between amphetamine and serotonin is only apparent in particular regions of the brain, such as the
mesocorticolimbic projection. Recent studies additionally postulate that amphetamine may indirectly alter the
behavior of glutamatergic pathways extending from the ventral tegmental area to the prefrontal cortex.[49]
Glutamatergic pathways are strongly correlated with increased excitability at the level of the synapse. Increased
extracellular concentrations of serotonin may thus modulate the excitatory activity of glutamatergic neurons.[49]
8
Amphetamine
9
The proposed ability of amphetamine to increase excitability of glutamatergic pathways may be of significance when
considering serotonin-mediated addiction.[49] An additional behavioral consequence may be the stereotyped
locomotor stimulation that occurs in response to amphetamine exposure.[38]
Other relevant neurotransmitters
Several other neurotransmitters have been linked to amphetamine activity. For instance, extracellular levels of
glutamate, the primary excitatory neurotransmitter in the brain, have been shown to increase upon exposure to
amphetamine. Consistent with other findings, this effect was found in the areas of the brain implicated in reward;
namely, the nucleus accumbens, striatum, and prefrontal cortex. Additionally, several studies demonstrate increased
levels of norepinephrine, a neurotransmitter related to adrenaline, in response to amphetamine. This is believed to
occur via reuptake blockage as well as via interactions with the norepinephrine neuronal transport carrier.[51] The
long-term effects of amphetamines use on neural development in children has not been well established.[52] Based on
a study in rats, amphetamine use during adolescence may impair adult working memory.[53]
Pharmacology
Chemical properties
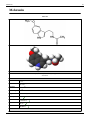
Amphetamine is a chiral compound. The racemic mixture can be
divided into its optical isomers: levo- and dextro-amphetamine.
Amphetamine is the parent compound of its own structural class,
comprising a broad range of psychoactive derivatives, from
empathogens, MDA (3,4-Methylenedioxyamphetamine) and MDMA
(3,4-Methylenedioxy-N-methamphetamine) known as ecstasy, to the
N-methylated form, methamphetamine known as 'meth', and to
decongestants such as ephedrine (EPH) . Amphetamine is a homologue
of phenethylamine.
Amphetamine molecular structure.
Methamphetamine has the same structure, with
the addition of a methyl group attached to the
nitrogen.
At first, the medical drug came as the salt racemic-amphetamine
sulfate (racemic-amphetamine contains both isomers in equal
amounts). Attention disorders are often treated using Adderall or a
generic equivalent, a formulation of mixed amphetamine and
dextroamphetamine salts that contain
•
•
•
•
1/4 dextro-amphetamine saccharate
1/4 dextro-amphetamine sulfate
1/4 (racemic amphetamine) aspartate monohydrate
1/4 (racemic amphetamine) sulfate
Pharmacodynamics
Amphetamine has been shown to both diffuse through the cell
membrane and travel via the dopamine transporter (DAT) to increase
concentrations of dopamine in the neuronal terminal.
A chart A comparing the chemical structures of
different amphetamine derivatives
Amphetamine, both as d-amphetamine (dextroamphetamine) and l-amphetamine (or a racemic mixture of the two
isomers), is believed to exert its effects by binding to the monoamine transporters and increasing extracellular levels
of the biogenic amines dopamine, norepinephrine (noradrenaline) and serotonin. It is hypothesized that
d-amphetamine acts primarily on the dopaminergic systems, while l-amphetamine is comparatively
norepinephrinergic (noradrenergic). The primary reinforcing and behavioral-stimulant effects of amphetamine,
Amphetamine
however, are linked to enhanced dopaminergic activity, primarily in the mesolimbic dopamine system.
Amphetamine and other amphetamine-type stimulants principally act to release dopamine into the synaptic cleft.
Amphetamine, unlike dopamine transporter inhibitor cocaine, acts as a substrate for DAT and slows reuptake by a
secondary acting mechanism through the phosphorylation of dopamine transporters.[54] A primary action of
amphetamine is mediated by vesicular monoamine transporters (VMATs); the transporters appear to provide a
channel through which dopamine and other transmitters can exit the vesicle to the cytosol. According to the "weak
base hypothesis" this is exacerbated by amphetamine acting to alkalinize the vesicle, which depletes the free energy
favoring vesicle accummlation so that transmitter is redistributed to the cytosol. Together, these actions cause the
release of dopamine, norepinephrine, and serotonin from monoamine vesicles, thereby increasing cytosolic
concentrations of transmitter. This increase in concentration assists in the "reverse transport" of dopamine via the
dopamine transporter (DAT) into the synapse.[55] In addition, amphetamine binds reversibly to the DATs and blocks
the transporter's ability to clear DA from the synaptic space. Amphetamine also acts in this way with norepinephrine
(noradrenaline) and to a lesser extent serotonin.
In addition, amphetamine binds to a group of receptors called Trace Amine Associated Receptors (TAAR).[56]
TAAR are a newly discovered receptor system which seems to be affected by a range of amphetamine-like
substances called trace amines.
History
Amphetamine was first synthesized in 1887 by the Romanian chemist Lazăr Edeleanu in Berlin, Germany.[57] He
named the compound phenylisopropylamine. It was one of a series of compounds related to the plant derivative
ephedrine, which had been isolated from Ma-Huang that same year by Nagayoshi Nagai.[58] No pharmacological use
was found for amphetamine until 1927, when pioneer psychopharmacologist Gordon Alles resynthesized and tested
it on himself, in search of an artificial replacement for ephedrine. From 1933 or 1934 Smith, Kline and French began
selling the volatile base form of the drug as an inhaler under the trade name Benzedrine, useful as a decongestant but
readily usable for other purposes.[59] One of the first attempts at using amphetamine as a scientific study was done
by M. H. Nathanson, a Los Angeles physician, in 1935. He studied the subjective effects of amphetamine in 55
hospital workers who were each given 20 mg of Benzedrine. The two most commonly reported drug effects were "a
sense of well being and a feeling of exhilaration" and "lessened fatigue in reaction to work".[60] During World War II
amphetamine was extensively used to combat fatigue and increase alertness in soldiers. After decades of reported
abuse, the FDA banned Benzedrine inhalers, and limited amphetamine to prescription use in 1965, but non-medical
use remained common. Amphetamine became a schedule II drug under the Controlled Substances Act in 1971.
The related compound methamphetamine was first synthesized from ephedrine in Japan in 1920 by chemist Akira
Ogata, via reduction of ephedrine using red phosphorus and iodine. The pharmaceutical Pervitin was a tablet of 3 mg
methamphetamine which was available in Germany from 1938 and widely used in the Wehrmacht, but by mid-1941
it became a controlled substance, partly because of the amount of time needed for a soldier to rest and recover after
use and partly because of abuse. For the rest of the war, military doctors continued to issue the drug, but less
frequently and with increasing discrimination.[61]
In 1997 and 1998,[62] [63] researchers at Texas A&M University claimed to have found amphetamine and
methamphetamine in the foliage of two Acacia species native to Texas, A. berlandieri and A. rigidula. Previously,
both of these compounds had been thought to be human inventions. These findings have never been duplicated, and
the analyses are believed by many biochemists to be the result of experimental error, and as such the validity of the
report has come into question. Alexander Shulgin, one of the most experienced biochemical investigators and the
discoverer of many new psychotropic substances, has tried to contact the Texas A&M researchers and verify their
findings. The authors of the paper have not responded; natural amphetamine remains an unconfirmed discovery.[64]
10
Amphetamine
Performance-enhancing use
Adderall, an amphetamine mixture, is used by some college and high-school students as a study and test-taking
aid.[65] Amphetamine works by increasing energy levels, concentration, and motivation, thus allowing students to
study for an extended period of time.
Amphetamine has been, and is still, used by militaries around the world. British troops used 72 million amphetamine
tablets in the second world war[66] and the RAF used so many that "Methedrine won the Battle of Britain" according
to one report.[67] American bomber pilots use amphetamine ("go pills") to stay awake during long missions. The
Tarnak Farm incident, in which an American F-16 pilot killed several friendly Canadian soldiers on the ground, was
blamed by the pilot on his use of amphetamine.[68] A nonjudicial hearing rejected the pilot's claim.
Amphetamine is also used by some professional,[69] collegiate[70] and high school[70] athletes for its strong stimulant
effect. Energy levels are perceived to be dramatically increased and sustained, which is believed to allow for more
vigorous and longer play. However, at least one study has found that this effect is not measurable.[71] The use of
amphetamine during strenuous physical activity can be extremely dangerous, especially when combined with
alcohol, and athletes have died as a result, for example, British cyclist Tom Simpson.
Amphetamine use has historically been especially common among Major League Baseball players and is usually
known by the slang term "greenies".[72] In 2006, the MLB banned the use of amphetamine. The ban is enforced
through periodic drug-testing. However, the MLB has received some criticism because the consequences for
amphetamine use are dramatically less severe than for anabolic steroid use, with the first offense bringing only a
warning and further testing.[73] [74] [75]
Amphetamine was formerly in widespread use by truck drivers[76] to combat symptoms of somnolence and to
increase their concentration during driving, especially in the decades prior to the signing by former president Ronald
Reagan of Executive Order 12564, which initiated mandatory random drug testing of all truck drivers and employees
of other DOT-regulated industries. Although implementation of the order on the trucking industry was kept to a
gradual rate in consideration of its projected effects on the national economy, in the decades following the order,
amphetamine and other drug abuse by truck drivers has since dropped drastically. (See also Truck
driver—Implementation of drug detection).
Detection in body fluids
Amphetamine is frequently measured in urine as part of a drug abuse testing program, in plasma or serum to confirm
a diagnosis of poisoning in hospitalized victims, or in whole blood to assist in the forensic investigation of a traffic
or other criminal violation or a case of sudden death. Techniques such as immunoassay may cross-react with a
number of sympathomimetics drugs, so chromatographic methods specific for amphetamine should be employed to
prevent false positive results. Chiral techniques may be employed to help distinguish the source of the drug, whether
obtained legally (via prescription) or illicitly, or possibly as a result of formation from a prodrug such as
lisdexamfetamine or selegiline. Chiral separation is needed to assess the possible contribution of l-methamphetamine
(Vicks Inhaler) toward a positive test result.[77] [78] [79]
11
Amphetamine
Society and culture
From the 1960s onward, amphetamine has been popular with many youth subcultures in Britain (and other parts of
the world) as a recreational drug. It has been commonly used by mods, skinheads, punks, goths, gangsters, and
casuals in all night soul and ska dances, punk concerts, basement shows and fighting on the terraces by casuals.
The hippie counterculture was very critical of amphetamines due to the behaviors they cause; in an interview with
the Los Angeles Free Press in 1965, beat writer Allen Ginsberg commented that "Speed is antisocial, paranoid
making, it's a drag... all the nice gentle dope fiends are getting screwed up by the real horror monster Frankenstein
speed freaks who are going round stealing and bad-mouthing everybody".[80]
In literature
The writers of the Beat Generation used amphetamine extensively, mainly under the Benzedrine brand name. Jack
Kerouac was a particularly avid user of amphetamine, which was said to provide him with the stamina needed to
work on his novels for extended periods of time.[81]
Scottish author Irvine Welsh often portrays drug use in his novels, though in one of his journalism works he
comments on how drugs (including amphetamine) have become part of consumerism and how his novels
Trainspotting and Porno reflect the changes in drug use and culture during the years that elapse between the two
texts.[82]
Amphetamine is frequently mentioned in the work of American journalist Hunter S. Thompson. Speed appears not
only amongst the inventory of drugs Thompson consumed for what could broadly be defined as recreational
purposes, but also receives frequent, explicit mention as an essential component of his writing toolkit,[83] such as in
his "Author's Note" in Fear and Loathing on the Campaign Trail '72.[84]
"One afternoon about three days ago [the publishers] showed up at my door with no warning, and loaded
about forty pounds of supplies into the room: two cases of Mexican beer, four quarts of gin, a dozen
grapefruits, and enough speed to alter the outcome of six Super Bowls. ... Meanwhile, [...] with the final
chapter still unwritten and the presses scheduled to start rolling in twenty-four hours . . . . unless
somebody shows up pretty soon with extremely powerful speed, there might not be a final chapter.
About four fingers of king-hell Crank would do the trick, but I am not optimistic."
In mathematics
Famous mathematician Paul Erdős took amphetamine, and once won a bet from his friend Ron Graham, who bet him
$500 that he could not stop taking the drug for a month.[85] Erdős won the bet, but complained during his abstinence
that mathematics had been set back by a month: "Before, when I looked at a piece of blank paper my mind was filled
with ideas. Now all I see is a blank piece of paper." After he won the bet, he promptly resumed his amphetamine
habit.
In music
Many songs have been written about amphetamine, for example in the track entitled "St. Ides Heaven" from
singer/songwriter, Elliott Smith's self-titled album. Semi Charmed Life by Third Eye Blind also references
amphetamine. Another blatant example would be the song simply labelled "Amphetamine" by Alternative rock band
Everclear, and the song "20 Dollar nose bleed" by the Pop-rock band Fall Out Boy. It has also influenced the
aesthetics of many rock'n'roll bands (especially in the garage rock, mod R&B, death rock, punk/hardcore, gothic rock
and extreme heavy metal genres). Hüsker Dü, Jesus and Mary Chain's and The Who were keen amphetamine users
early in their existence. Land Speed Record is an allusion to Hüsker Dü's amphetamine use. Amphetamine was
widely abused in the 1980s underground punk-rock scene. Pop-punk band NOFX have incorporated references to
Amphetamines and other stimulants, the two most obvious being the song "Three on Speed" from the "Surfer" 8" LP
12
Amphetamine
(in reference to the three guys being on Amphetamine while recording the album), and earlier the album "The
Longest Line" is in reference to a "line" of Amphetamine ready for insufflation. The Rolling Stones referenced the
drug in their song "Can't You Hear Me Knocking" on the album Sticky Fingers ("Y'all got cocaine eyes / Yeah, ya
got speed-freak jive now"). Lou Reed refers explicitly to the drug on his album Berlin, in the song "How Do You
Think It Feels?". Reed's band The Velvet Underground, a creation of Andy Warhol's Factory Years, was fueled by
amphetamines, as well as naming their second album White Light/White Heat after the drug and making reference to
the song in "Sister Ray.".
Many rock'n'roll bands have named themselves after amphetamine and drug slang surrounding it. For example Mod
revivalists, The Purple Hearts named themselves after the amphetamine tablets popular with mods during the 1960s,
as did the Australian band of the same name during the mid-1960s. The Amphetameanies, a ska-punk band, are also
named after amphetamine, but also imitate its effects. Dexy's Midnight Runners, of number one hit "Come On
Eileen", are named after Dexedrine. Motörhead derived their name from the British slang for 'speed freak' and
Motorhead's Lemmy Kilmister is a long-term user of speed.
In film
Producer David O. Selznick was an amphetamine user, and would often dictate long and rambling memos under the
influence of amphetamine to his directors.[86] The documentary Shadowing The Third Man relates that Selznick
introduced The Third Man director Carol Reed to the use of amphetamine, which allowed Reed to bring the picture
in below budget and on schedule by filming nearly 22 hours at a time.[87]
Garrett Scott's documentary Cul-de-Sac: A Suburban War Story has a brief history of the manufacture and spread of
amphetamine, and of its effects.[88]
In the film Requiem for a Dream, Ellen Burstyn portrays Sara Goldfarb, an elderly widow who becomes addicted to
weight-loss amphetamine pills. After suffering from amphetamine psychosis, she is hospitalized against her will,
undergoes electro-convulsive therapy, and later on was confined at a mental asylum.[89]
The title of the 2009 movie Amphetamine plays on the double meaning of the word in Chinese - besides the name for
the drug it also means 'isn't this his fate?' which figuratively ties to the movie's plot. The word is transliterated as 安
非 他 命 - "ān fēi tā mìng" - and as commonly happens with transliteration of non-Chinese terms each character has
independent meaning as an individual unrelated word.
In the 1972 film Ciao! Manhattan, Edie Sedgwick portrays Susan Superstar, a fictional version of herself, who
discusses her addiction with drugs, mainly amphetamines. In one particular scene, she talks about the exhilaration of
her drug addiction in the infamous "Speed Monologue".[90]
In the 2005 Oscar winner Walk the Line Joaquin Phoenix portrays John Cash, an uprising star in the music industry.
He becomes addicted to amphetamines and eventually gets caught trying to cross borders with them.
In the film Empire Records Liv Tyler's character admits to having an amphetamine addiction.
Legal status
• In the United Kingdom, amphetamines are regarded as Class B drugs. The maximum penalty for unauthorized
possession is five years in prison and an unlimited fine. The maximum penalty for illegal supply is 14 years in
prison and an unlimited fine. Methamphetamine has recently been reclassified to Class A, penalties for possession
of which are more severe (7 years in prison and an unlimited fine).[91]
• In the Netherlands, amphetamine and methamphetamine are List I drugs of the Opium Law, but the dextro isomer
of amphetamine is indicated for ADD/ADHD and narcolepsy and available for prescription as 5 and 10 mg
generic tablets, and 5 and 10 mg gel capsules.
• In the United States, amphetamine and methamphetamine are Schedule II drugs, classified as CNS (central
nervous system) stimulants.[92] A Schedule II drug is classified as one that has a high potential for abuse, has a
13
Amphetamine
currently-accepted medical use and is used under severe restrictions, and has a high possibility of severe
psychological and physiological dependence.
• In Canada, possession of amphetamines is a criminal offence under Schedule III of the Controlled Drugs and
Substances Act, with a maximum penalty for repeat offenders of fines of up to $2,000, imprisonment for up to
one year, or both.[93]
Internationally, amphetamine is a Schedule II drug under the Convention on Psychotropic Substances.[94]
Prodrugs
A number of substances have been shown to produce amphetamine and/or methamphetamine as metabolites,
including amfecloral, amphetaminil, benzphetamine, clobenzorex, dimethylamphetamine, ethylamphetamine,
famprofazone, fencamine, fenethylline, fenproporex, furfenorex, lisdexamfetamine, mefenorex, mesocarb,
prenylamine, propylamphetamine, and selegiline, among others.[95] [96] These compounds may produce positive
results for amphetamine on drug tests.[95] [96]
Derivatives
Amphetamine derivatives are a class of potent drugs that act by increasing levels of dopamine and norepinephrine in
the brain, inducing euphoria.[97] [98] [99] The class includes prescription CNS drugs commonly used to treat
attention-deficit hyperactivity disorder (ADHD). It is also used to treat symptoms of traumatic brain injury (TBI) and
the daytime drowsiness symptoms of narcolepsy, postural orthostatic tachycardia syndrome (POTS) and chronic
fatigue syndrome (CFS).
References and notes
[1] http:/ / www. nlm. nih. gov/ cgi/ mesh/ 2009/ MB_cgi?term=300-62-9& rn=1
[2] http:/ / toolserver. org/ ~magnus/ cas. php?language=en& cas=405-41-4& title=
[3] http:/ / www. whocc. no/ atc_ddd_index/ ?code=N06BA01
[4] http:/ / pubchem. ncbi. nlm. nih. gov/ summary/ summary. cgi?cid=3007
[5] http:/ / www. drugbank. ca/ drugs/ DB00182
[6] http:/ / www. chemspider. com/ Chemical-Structure. 13852819
[7] http:/ / fdasis. nlm. nih. gov/ srs/ srsdirect. jsp?regno=CK833KGX7E
[8] http:/ / www. kegg. jp/ entry/ D07445
[9] https:/ / www. ebi. ac. uk/ chembldb/ index. php/ compound/ inspect/ CHEMBL405
[10] http:/ / www. emolecules. com/ cgi-bin/ search?t=ex& q=NC%28C%29Cc1ccccc1
[11] http:/ / pubchem. ncbi. nlm. nih. gov/ search/ ?smarts=NC%28C%29Cc1ccccc1
[12] Miranda-G E, Sordo M, Salazar AM (2007). "Determination of amphetaminoe, methamphetamine, and hydroxyamphetamine derivatives in
urine by gas chromatography-mass spectrometry and its relation to CYP2D6 phenotype of drug users" (http:/ / openurl. ingenta. com/ content/
nlm?genre=article& issn=0146-4760& volume=31& issue=1& spage=31& aulast=Miranda-G). J Anal Toxicol 31 (1): 31–6. PMID 17389081.
.
[13] http:/ / en. wikipedia. org/ w/ index. php?& diff=cur& oldid=398687910
[14] Drug Test FAQ | Craig Medical Distribution, Inc. (http:/ / www. craigmedical. com/ drug_test_faq. htm)
[15] European Monitoring Centre for Drugs and Drug Addiction (2008) (PDF). Annual report: the state of the drugs problem in Europe (http:/ /
www. emcdda. europa. eu/ attachements. cfm/ att_64227_EN_EMCDDA_AR08_en. pdf). Luxembourg: Office for Official Publications of the
European Communities. p. 48. ISBN 978-92-9168-324-6. .
[16] Nutt, D; King, LA; Saulsbury, W; Blakemore, C (2007). "Development of a rational scale to assess the harm of drugs of potential misuse.".
Lancet 369 (9566): 1047–53. doi:10.1016/S0140-6736(07)60464-4. PMID 17382831.
[17] Erowid Amphetamines Vault | Effects (http:/ / www. erowid. org/ chemicals/ amphetamines/ amphetamines_effects. shtml)
[18] Amphetamine; Facts | Alcoholism and Drug Addiction Research Foundation, Toronto Canada (http:/ / www. xs4all. nl/ ~4david/ amphetam.
html)
[19] Amphetamines | Better Health Channel (http:/ / www. betterhealth. vic. gov. au/ bhcv2/ bhcarticles. nsf/ pages/ Amphetamines)
[20] Dextroamphetamine (Oral Route) | MayoClinic.com (http:/ / www. mayoclinic. com/ health/ drug-information/
DR602659#C5B63D57-E7FF-0DBD-1B31F9CC4EA63178)
14
Amphetamine
[21] Vitiello B (2008). "Understanding the risk of using medications for attention deficit hyperactivity disorder with respect to physical growth
and cardiovascular function". Child Adolesc Psychiatr Clin N Am 17 (2): 459–74, xi. doi:10.1016/j.chc.2007.11.010. PMC 2408826.
PMID 18295156.
[22] Ricaurte GA, Mechan AO, Yuan J, et al. (October 2005). "Amphetamine treatment similar to that used in the treatment of adult
attention-deficit/hyperactivity disorder damages dopaminergic nerve endings in the striatum of adult nonhuman primates" (http:/ /
pharmacology. ucsd. edu/ graduate/ courseinfo/ documents/ ricaurteadhd. pdf) (PDF). J. Pharmacol. Exp. Ther. 315 (1): 91–8.
doi:10.1124/jpet.105.087916. PMID 16014752. . Retrieved 28 March 2011.
[23] Erowid Amphetamines Vault: Effects (http:/ / www. erowid. org/ chemicals/ amphetamines/ amphetamines_effects. shtml)
[24] Amphetamines | Merck Sharp & Dohme Corp. (http:/ / www. merck. com/ mmpe/ sec15/ ch198/ ch198k. html)
[25] Symptoms of Amphetamine withdrawal | WrongDiagnosis.com (http:/ / www. wrongdiagnosis. com/ a/ amphetamine_withdrawal/
symptoms. htm#symptom_list)
[26] eMedTV | Dextroamphetamine Withdrawals (http:/ / sleep. emedtv. com/ dextroamphetamine/ dextroamphetamine-withdrawals. html)
[27] Drug Abuse Help | Dexedrine Information (http:/ / www. drugabusehelp. com/ drugs/ dexedrine/ )
[28] Amphetamine Disease Interactions - Drugs.com (http:/ / www. drugs. com/ disease-interactions/ amphetamine. html)
[29] "ADDERALL XR capsule" (http:/ / www. accessdata. fda. gov/ drugsatfda_docs/ label/ 2004/ 021303s005lbl. pdf) (PDF). 2005. . Retrieved
2009-07-24.
[30] "Amphetamines: Drug Use and Abuse: Merck Manual Home Edition" (http:/ / web. archive. org/ web/ 20070217053619/ http:/ / www.
merck. com/ mmhe/ sec07/ ch108/ ch108g. html). Merck. Archived from the original (http:/ / www. merck. com/ mmhe/ sec07/ ch108/
ch108g. html) on February 17, 2007. . Retrieved February 28, 2007.
[31] Jones S, Kornblum JL, Kauer JA (August 2000). "Amphetamine blocks long-term synaptic depression in the ventral tegmental area" (http:/ /
www. jneurosci. org/ cgi/ pmidlookup?view=long& pmid=10908593). J. Neurosci. 20 (15): 5575–80. PMID 10908593. .
[32] Moore KE (June 1977). "The actions of amphetamine on neurotransmitters: a brief review". Biol. Psychiatry 12 (3): 451–62. PMID 17437.
[33] Del Arco A, González-Mora JL, Armas VR, Mora F (July 1999). "Amphetamine increases the extracellular concentration of glutamate in
striatum of the awake rat: involvement of high affinity transporter mechanisms". Neuropharmacology 38 (7): 943–54.
doi:10.1016/S0028-3908(99)00043-X. PMID 10428413.
[34] Drevets WC, Gautier C, Price JC (January 2001). "Amphetamine-induced dopamine release in human ventral striatum correlates with
euphoria". Biol. Psychiatry 49 (2): 81–96. doi:10.1016/S0006-3223(00)01038-6. PMID 11164755.
[35] Wise, RA. “Brain reward circuitry and addiction.” Program and abstracts of the American Society of Addiction Medicine 2003 The State of
the Art in Addiction Medicine; October 30-November 1, 2003; Washington, DC. Session
[36] Sulzer D, Chen TK, Lau YY, Kristensen H, Rayport S, Ewing A (May 1995). "Amphetamine redistributes dopamine from synaptic vesicles
to the cytosol and promotes reverse transport" (http:/ / www. jneurosci. org/ cgi/ pmidlookup?view=long& pmid=7751968). J. Neurosci. 15 (5
Pt 2): 4102–8. PMID 7751968. .
[37] Sulzer, D. (2011). "How Addictive Drugs Disrupt Presynaptic Dopamine Neurotransmission". Neuron 69 (4): 628–649.
doi:10.1016/j.neuron.2011.02.010. PMC 3065181. PMID 21338876.
[38] Kuczenski R, Segal DS (May 1997). "Effects of methylphenidate on extracellular dopamine, serotonin, and norepinephrine: comparison
with amphetamine". J. Neurochem. 68 (5): 2032–7. doi:10.1046/j.1471-4159.1997.68052032.x. PMID 9109529.
[39] Rothman RB, Baumann MH (August 2006). "Balance between dopamine and serotonin release modulates behavioral effects of
amphetamine-type drugs". Ann. N. Y. Acad. Sci. 1074: 245–60. doi:10.1196/annals.1369.064. PMID 17105921.
[40] Johnson LA, Guptaroy B, Lund D, Shamban S, Gnegy ME (March 2005). "Regulation of amphetamine-stimulated dopamine efflux by
protein kinase C beta". J. Biol. Chem. 280 (12): 10914–9. doi:10.1074/jbc.M413887200. PMID 15647254.
[41] Kahlig KM, Binda F, Khoshbouei H (March 2005). "Amphetamine induces dopamine efflux through a dopamine transporter channel". Proc.
Natl. Acad. Sci. U.S.A. 102 (9): 3495–500. doi:10.1073/pnas.0407737102. PMC 549289. PMID 15728379.
[42] "A mechanism for amphetamine-induced dopamine overload". PLoS Biology 2 (3): e87. 2004. doi:10.1371/journal.pbio.0020087.
PMC 368179.
[43] Sulzer, D.; Chen, T.; Lau, Y.; Kristensen, H.; Rayport, S.; Ewing, A. (1995). "Amphetamine redistributes dopamine from synaptic vesicles
to the cytosol and promotes reverse transport". The Journal of neuroscience : the official journal of the Society for Neuroscience 15 (5 Pt 2):
4102–4108. PMID 7751968.
[44] Mosharov, E. V.; Larsen, K. E.; Kanter, E.; Phillips, K. A.; Wilson, K.; Schmitz, Y.; Krantz, D. E.; Kobayashi, K. et al. (2009). "Interplay
between Cytosolic Dopamine, Calcium, and α-Synuclein Causes Selective Death of Substantia Nigra Neurons". Neuron 62 (2): 218–229.
doi:10.1016/j.neuron.2009.01.033. PMC 2677560. PMID 19409267.
[45] Sulzer, D.; Rayport, S. (1990). "Amphetamine and other psychostimulants reduce pH gradients in midbrain dopaminergic neurons and
chromaffin granules: A mechanism of action". Neuron 5 (6): 797–808. PMID 2268433.
[46] Larsen, K.; Fon, E.; Hastings, T.; Edwards, R.; Sulzer, D. (2002). "Methamphetamine-induced degeneration of dopaminergic neurons
involves autophagy and upregulation of dopamine synthesis". The Journal of neuroscience : the official journal of the Society for
Neuroscience 22 (20): 8951–8960. PMID 12388602.
[47] Sulzer, D.; Sonders, M.; Poulsen, N.; Galli, A. (2005). "Mechanisms of neurotransmitter release by amphetamines: a review". Progress in
neurobiology 75 (6): 406–433. doi:10.1016/j.pneurobio.2005.04.003. PMID 15955613.
[48] Schmitz, Y.; Lee, C.; Schmauss, C.; Gonon, F.; Sulzer, D. (2001). "Amphetamine distorts stimulation-dependent dopamine overflow:
Effects on D2 autoreceptors, transporters, and synaptic vesicle stores". The Journal of neuroscience : the official journal of the Society for
15
Amphetamine
Neuroscience 21 (16): 5916–5924. PMID 11487614.
[49] Jones S, Kauer JA (November 1999). "Amphetamine depresses excitatory synaptic transmission via serotonin receptors in the ventral
tegmental area" (http:/ / www. jneurosci. org/ cgi/ pmidlookup?view=long& pmid=10559387). J. Neurosci. 19 (22): 9780–7.
PMID 10559387. .
[50] Hilber B, Scholze P, Dorostkar MM (November 2005). "Serotonin-transporter mediated efflux: a pharmacological analysis of amphetamines
and non-amphetamines". Neuropharmacology 49 (6): 811–9. doi:10.1016/j.neuropharm.2005.08.008. PMID 16185723.
[51] Florin SM, Kuczenski R, Segal DS (August 1994). "Regional extracellular norepinephrine responses to amphetamine and cocaine and
effects of clonidine pretreatment". Brain Res. 654 (1): 53–62. doi:10.1016/0006-8993(94)91570-9. PMID 7982098.
[52] RxList | Adderall (http:/ / www. rxlist. com/ adderall-drug. htm)
[53] "Amphetamine use in adolescence may impair adult working memory" (http:/ / news. illinois. edu/ news/ 09/ 1021amphetamine. html). .
Retrieved 2009-10-22.
[54] WikiAnswers google cached page: 'Does Namenda memantine work in preventing tolerance to adderall ADD amphetamine type drugs?'
(http:/ / 74. 125. 95. 132/ search?q=cache:BaDklpuwbTQJ:wiki. answers. com/ Q/
Does_Namenda_memantine_work_in_preventing_tolerance_to_adderall_ADD_amphetamine_type_drugs& hl=en& ct=clnk& cd=10& gl=us)
[55] Sulzer D, Sonders MS, Poulsen NW, Galli A (April 2005). "Mechanisms of neurotransmitter release by amphetamines: a review". Prog.
Neurobiol. 75 (6): 406–33. doi:10.1016/j.pneurobio.2005.04.003. PMID 15955613.
[56] Reese EA, Bunzow JR, Arttamangkul S, Sonders MS, Grandy DK (April 2007). "Trace amine-associated receptor 1 displays
species-dependent stereoselectivity for isomers of methamphetamine, amphetamine, and para-hydroxyamphetamine" (http:/ / jpet.
aspetjournals. org/ cgi/ content/ abstract/ 321/ 1/ 178). J Pharmacol Exp Ther. 321 (1): 178–86. doi:10.1124/jpet.106.115402.
PMID 17218486. .
[57] Edeleano L (1887). "Ueber einige Derivate der Phenylmethacrylsäure und der Phenylisobuttersäure". Berichte der deutschen chemischen
Gesellschaft 20 (1): 616–622. doi:10.1002/cber.188702001142.
[58] Shulgin, Alexander; Shulgin, Ann (1992). "6 – MMDA". PiHKAL. Berkeley, California: Transform Press. p. 39. ISBN 0-9630096-0-5.
[59] Rasmussen N (July 2006). "Making the first anti-depressant: amphetamine in American medicine, 1929–1950". J Hist Med Allied Sci 61 (3):
288–323. doi:10.1093/jhmas/jrj039. PMID 16492800.
[60] Iverson, Leslie. Speed, Ecstacy, Ritalin: the science of amphetamines. Oxford, New York. Oxford University Press, 2006.
[61] Rasmussen, Nicolas (2008). "Ch. 4". On Speed: The Many Lives of Amphetamine. New York, New York: New York University Press.
ISBN 0-8147-7601-9.
[62] Clement B.A., Goff C.M., Forbes T.D.A. (1998). "Toxic amines and alkaloids from Acacia rigidula". Phytochemistry 49 (5): 1377–1380.
doi:10.1016/S0031-9422(97)01022-4.
[63] Clement B.A., Goff C.M., Forbes T.D.A. (1997). "Toxic amines and alkaloids from Acacia berlandieri". Phytochemistry 46 (2): 249–254.
doi:10.1016/S0031-9422(97)00240-9.
[64] Ask Dr. Shulgin Online: Acacias and Natural Amphetamine (http:/ / www. cognitiveliberty. org/ shulgin/ adsarchive/ acacia. htm)
[65] Twohey, Megan (2006-03-25). "Pills become an addictive study aid" (http:/ / web. archive. org/ web/ 20070815200239/ http:/ / www.
jsonline. com/ story/ index. aspx?id=410902). JS Online. Archived from the original (http:/ / www. jsonline. com/ story/ index.
aspx?id=410902) on 2007-08-15. . Retrieved 2007-12-02.
[66] De Mondenard, Dr Jean-Pierre: Dopage, l'imposture des performances, Chiron, France, 2000
[67] Grant, D.N.W.; Air Force, UK, 1944
[68] "Air force rushes to defend amphetamine use" (http:/ / www. theage. com. au/ articles/ 2003/ 01/ 17/ 1042520778665. html). The Age.
January 18, 2003. . Retrieved 26 January 2009.
[69] Yesalis, Charles E.; Bahrke, Michael (2005-12). "Anabolic Steroid and Stimulant Use in North American Sport between 1850 and 1980"
(http:/ / www. informaworld. com/ smpp/ content~content=a727721070~db=all). Sport in History 25 (3): 434–451.
doi:10.1080/17460260500396251. . Retrieved 2007-12-02.
[70] National Collegiate Athletic Association (2006-01) (PDF), NCAA Study of Substance Use Habits of College Student-Athletes (http:/ / www1.
ncaa. org/ membership/ ed_outreach/ health-safety/ drug_ed_progs/ 2005/ DrugStudy2005_ExecutiveSummary. pdf), National Collegiate
Athletic Association, pp. 2–4, 11–13, , retrieved 2007-12-02
[71] Margaria, R; Aghemo, P.; Rovelli, E. (1964-07-01). "The effect of some drugs on the maximal capacity of athletic performance in man".
European Journal of Applied Physiology 20 (4): 281–287. doi:10.1007/BF00697020. PMID 14252788.
[72] Frias, Carlos (2006-04-02). "Baseball and amphetamines" (http:/ / www. palmbeachpost. com/ sports/ content/ sports/ epaper/ 2006/ 04/ 02/
PBP_AMPHET_0402. html). Palm Beach Post. . Retrieved 2007-12-02.
[73] Kreidler, Mark (2005-11-15). "Baseball finally brings amphetamines into light of day" (http:/ / sports. espn. go. com/ mlb/ columns/
story?columnist=kreidler_mark& id=2225013). ESPN.com. . Retrieved 2007-12-02.
[74] Klobuchar, Jim (2006-03-31). "Can baseball make a clean sweep?" (http:/ / www. csmonitor. com/ 2006/ 0331/ p12s02-alsp. html).
Christian Science Monitor. . Retrieved 2007-12-02.
[75] Associated Press (2007-01-18). "MLB owners won't crack down on 'greenies'" (http:/ / www. msnbc. msn. com/ id/ 16691245/ ).
MSNBC.com. . Retrieved 2007-12-02.
[76] Lund, Adrian K; David F. Preusser, Richard D. Blomberg, Allan F. Williams, J. Michael Walsh (1989). "Drug Use by Tractor-Trailer
Drivers" (http:/ / bib1lp1. rz. tu-bs. de/ docportal/ servlets/ MCRFileNodeServlet/ DocPortal_derivate_00002043/ 091.
pdf?hosts=local#page=54). In Steven W. Gust (ed.). Drugs in the Workplace: Research and Evaluation Data. National Institute on Drug
16
Amphetamine
Abuse Research. Rockville, MD: National Institute on Drug Abuse. pp. 47–67. . Retrieved 2007-12-02. "This study has provided the first
objective data regarding the use of potentially abusive drugs by tractor-trailer drivers... Prescription stimulants, such as amphetamine,
methamphetamine, and phentermine were found in 5 percent of the [317] drivers [who participated in the study], often in combination with
similar but less potent stimulants, such as phenylpropanolamine. Nonprescription stimulants were detected in 12 percent of the drivers, about
half of whom gave no medical explanation for their presence... One limitation of these findings is that 12 percent of the randomly selected
drivers refused to participate in the study or provided insufficient urine and blood for testing; the distribution of drugs among these 42 drivers
is unknown... Finally, the results apply to tractor-trailer drivers operating on a major east-west interstate route in Tennessee. Drug incidence
among other truck-driver populations are unknown and may be higher or lower than reported here. (64)"
[77] Verstraete AG, Heyden FV (2005). "Comparison of the sensitivity and specificity of six immunoassays for the detection of amphetamines in
urine". J Anal Toxicol 29 (5): 359–64. PMID 16105261.
[78] Paul BD, Jemionek J, Lesser D, Jacobs A, Searles DA (September 2004). "Enantiomeric separation and quantitation of (+/-)-amphetamine,
(+/-)-methamphetamine, (+/-)-MDA, (+/-)-MDMA, and (+/-)-MDEA in urine specimens by GC-EI-MS after derivatization with (R)-(-)- or
(S)-(+)-alpha-methoxy-alpha-(trifluoromethy)phenylacetyl chloride (MTPA)". J Anal Toxicol 28 (6): 449–55. PMID 15516295.
[79] R. Baselt, Disposition of Toxic Drugs and Chemicals in Man, 8th edition, Biomedical Publications, Foster City, CA, 2008, pp. 83-86.
[80] Brecher, Edward M.; the editors of Consumer Reports Magazine (1972). "How speed was popularized" (http:/ / www. druglibrary. org/
schaffer/ library/ studies/ cu/ cu38. html). The Consumers Union Report on Licit and Illicit Drugs. Schaffer Drug Library. . Retrieved 1 June
2010.
[81] Gyenis, Attila (1997). "Forty Years of On the Road 1957–1997" (http:/ / web. archive. org/ web/ 20080214171739/ http:/ / www.
wordsareimportant. com/ ontheroad. htm). Archived from the original (http:/ / www. wordsareimportant. com/ ontheroad. htm) on 14 February
2008. . Retrieved 18 March 2008.
[82] Welsh, Irvine (2006-08-10). "Drug Cultures in Trainspotting and Porno" (http:/ / www. irvinewelsh. net/ journalism. aspx?jid=25).
irvinewelsh.net. . Retrieved 2008-10-13.
[83] Carr, David (29 June 2008). "Fear and Loathing on a Documentary Screen" (http:/ / www. nytimes. com/ 2008/ 06/ 29/ movies/ 29carr.
html) (in en-US). New York Times. pp. AR7. . Retrieved 18 March 2009.
[84] Thompson, Hunter S. (1973). Fear and Loathing on the Campaign Trail '72. New York: Warner Books. pp. 15–16, 21.
ISBN 0-446-31364-5.
[85] Hill, J. Paul Erdos, Mathematical Genius, Human (In That Order) (http:/ / www. untruth. org/ ~josh/ math/ Paul Erdös bio-rev2. pdf)
[86] Memo From David O. Selznick, http:/ / www. amazon. com/ Memo-David-Selznick-Memorandums-Autobiographical/ dp/ 0375755314
[87] Shadowing the Third Man, http:/ / www. imdb. com/ title/ tt0429086/
[88] Cul-de-Sac, http:/ / www. imdb. com/ title/ tt0317273/
[89] Resurrection (1980) - IMDb (http:/ / www. imdb. com/ title/ tt0081414/ news)
[90] (http:/ / www. austinchronicle. com/ gyrobase/ Issue/ story?oid=oid:182251)
[91] "Class A, B and C drugs" (http:/ / www. homeoffice. gov. uk/ drugs/ drugs-law/ Class-a-b-c/ ). . Retrieved 2007-07-23.
[92] "Trends in Methamphetamine/Amphetamine Admissions to Treatment: 1993–2003" (http:/ / www. oas. samhsa. gov/ 2k6/ methTX/
methTX. htm). Substance Abuse and Mental Health Services Administration. . Retrieved February 28, 2007.
[93] "Straight Facts About Drugs & Drug Abuse" (http:/ / www. hc-sc. gc. ca/ hc-ps/ pubs/ adp-apd/ straight_facts-faits_mefaits/
tables-tableaux-eng. php). Health Canada. . Retrieved April 23, 2011.
[94] "List of psychotropic substances under international control" (http:/ / www. incb. org/ pdf/ e/ list/ green. pdf) (PDF). International Narcotics
Control Board. . Retrieved November 19, 2005.
[95] Musshoff F (February 2000). "Illegal or legitimate use? Precursor compounds to amphetamine and methamphetamine" (http:/ /
informahealthcare. com/ doi/ abs/ 10. 1081/ DMR-100100562). Drug Metabolism Reviews 32 (1): 15–44. doi:10.1081/DMR-100100562.
PMID 10711406. .
[96] Cody JT (May 2002). "Precursor medications as a source of methamphetamine and/or amphetamine positive drug testing results" (http:/ /
meta. wkhealth. com/ pt/ pt-core/ template-journal/ lwwgateway/ media/ landingpage. htm?issn=1076-2752& volume=44& issue=5&
spage=435). Journal of Occupational and Environmental Medicine / American College of Occupational and Environmental Medicine 44 (5):
435–50. PMID 12024689. .
[97] Drevets, W et al (2001). "Amphetamine-Induced Dopamine Release in Human Ventral Striatum Correlates with Euphoria" (http:/ /
www-psych. stanford. edu/ ~knutson/ rab/ drevets01. pdf). Psychiatry 49: 81–96. . Retrieved 23 May 2009.
[98] Rang and Dale, Pharmacology
[99] Schep LJ, Slaughter RJ, Beasley DM (August 2010). "The clinical toxicology of metamfetamine". Clinical Toxicology (Philadelphia, Pa.)
48 (7): 675–94. doi:10.3109/15563650.2010.516752. ISSN 1556-3650. PMID 20849327.
17
Amphetamine
External links
• CID 5826 (http://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?cid=5826) from PubChem
(D-form—dextroamphetamine)
• CID 3007 (http://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?cid=3007) from PubChem (L-form and
D, L-forms)
• CID 32893 (http://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?cid=32893) from PubChem
(L-form—Levamphetamine or L-amphetamine)
• List of 504 Compounds Similar to Amphetamine (PubChem) (http://www.ncbi.nlm.nih.gov/sites/
entrez?Db=pccompound&DbFrom=pccompound&Cmd=Link&LinkName=pccompound_pccompound&
LinkReadableName=Similar Compounds&IdsFromResult=3007&ordinalpos=1&itool=EntrezSystem2.
PEntrez.Pccompound.Pccompound_ResultsPanel.Pccompound_RVDocSum)
• EMCDDA drugs profile: Amphetamine (2007) (http://www.emcdda.europa.eu/?nnodeid=25479)
• Drugs.com - Amphetamine (http://www.drugs.com/Amphetamine)
• Asia & Pacific Amphetamine-Type Stimulants Information Centre (http://www.apaic.org)
• U.S. National Library of Medicine: Drug Information Portal - Amphetamine (http://druginfo.nlm.nih.gov/
drugportal/dpdirect.jsp?name=Amphetamine)
Ataractive
An ataractive is a type of drug which diminishes hallucinations in patients exhibiting them.[1]
Examples include azacyclonol[1] and atypical antipsychotics.
References
[1] BRAUN DL, BROWN BB, FELDMAN RG (October 1956). "The pharmacologic activity of alpha-(4-piperidyl)-benzhydrol hydrochloride
(azacyclonol hydrochloride); an ataractive agent" (http:/ / jpet. aspetjournals. org/ cgi/ pmidlookup?view=long& pmid=13368052). The
Journal of Pharmacology and Experimental Therapeutics 118 (2): 153–61. PMID 13368052. .
18
Cathinone
19
Cathinone
Cathinone
Systematic (IUPAC) name
(S)-2-amino-1-phenyl-1-propanone
Identifiers
CAS number
71031-15-7
ATC code
None
PubChem
CID 62258
ChemSpider
56062
KEGG
C08301
[1]
[2]
[3]
[4]
Chemical data
Formula
C H NO
Mol. mass
149.19 g/mol
SMILES
eMolecules
9
11
[5]
& PubChem
[6]
Therapeutic considerations
Pregnancy cat. ?
Legal status
Schedule III (CA) ? (UK) Schedule I (US)
(what is this?) (verify)
[7]
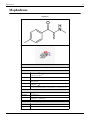
Cathinone, or Benzoylethanamine, is a monoamine alkaloid found in the shrub Catha edulis (khat) and is
chemically similar to ephedrine, cathine and other amphetamines. Cathinone induces the release of dopamine from
striatal preparations that are prelabelled either with dopamine or its precursors.[8] It is probably the main contributor
to the stimulant effect of Catha edulis. Cathinone differs from many other amphetamines in that it has a ketone
functional group. Other amphetamines that share this structure include the antidepressant bupropion and the
stimulant methcathinone, among others.
Cathinone
20
Internationally, cathinone is a Schedule I drug under the Convention on Psychotropic Substances.[9] Circa 1993, the
DEA added cathinone to the Controlled Substances Act's Schedule I.
The sale of khat is legal in Israel (although synthetic cathinone is not), and also in Oman, in Yemen, in United
Kingdom and in the Horn of Africa.
Chemistry
The molecular structure of cathinone.
Cathinone is structurally related to methcathinone, in much the
same way as amphetamine is related to methamphetamine.
Cathinone differs from amphetamine by possessing a ketone
oxygen atom (C=O) on the β (beta) position of the side chain. The
corresponding alcohol compound cathine is a less powerful
stimulant. The biophysiological conversion from cathinone to
cathine is to blame for the depotentiation of khat leaves over time.
Fresh leaves have a greater ratio of cathinone to cathine than dried
ones, therefore having more psychoactive effects.
Cathinone can be extracted from Catha edulis, or synthesized from
α-bromopropiophenone (which is easily made from propiophenone).
Toxic effects
Excessive cathinone usage can cause loss of appetite, anxiety, irritability, insomnia, hallucinations and panic attacks.
Chronic abusers are at risk of developing personality disorders and of sustaining myocardial infarction. Persons
driving under the influence of the drug have had their serum or urine tested for the presence of cathinone and
norephedrine, a major metabolite.[10]
References
[1]
[2]
[3]
[4]
[5]
[6]
[7]
[8]
http:/ / www. nlm. nih. gov/ cgi/ mesh/ 2009/ MB_cgi?term=71031-15-7& rn=1
http:/ / pubchem. ncbi. nlm. nih. gov/ summary/ summary. cgi?cid=62258
http:/ / www. chemspider. com/ Chemical-Structure. 56062
http:/ / www. kegg. jp/ entry/ C08301
http:/ / www. emolecules. com/ cgi-bin/ search?t=ex& q=O%3DC%28c1ccccc1%29%5BC%40%40H%5D%28N%29C
http:/ / pubchem. ncbi. nlm. nih. gov/ search/ ?smarts=O%3DC%28c1ccccc1%29%5BC%40%40H%5D%28N%29C
http:/ / en. wikipedia. org/ w/ index. php?& diff=cur& oldid=399710764
Kalix, Peter (February 1981). "Cathinone, an alkaloid from khat leaves with an amphetamine-like releasing effect" (http:/ / www.
springerlink. com/ content/ n7v28h68627245j0/ ?p=95742099c2304cc9b83799274e8bbcdc& pi=2). Psychopharmacology 74 (3): 269–70.
doi:10.1007/BF00427108. PMID 6791236. . Retrieved 2008-02-11.
[9] List of psychotropic substances under international control (http:/ / www. incb. org/ pdf/ e/ list/ green. pdf)
[10] R. Baselt, Disposition of Toxic Drugs and Chemicals in Man, 8th edition, Biomedical Publications, Foster City, CA, 2008, pp. 250-252.
External links
• Erowid Cathinone Vault (http://www.erowid.org/chemicals/cathinone/)
• Cathinone Popularity Soars in Israel (http://www.guardian.co.uk/israel/Story/0,2763,1296958,00.html)
Dextrorphan
21
Dextrorphan
Dextrorphan
Systematic (IUPAC) name
(+)-17-methyl- 9a,13a,14a-morphinan- 3-ol
Identifiers
[1]
CAS number
125-73-5
ATC code
None
PubChem
CID 5360697
[2]
Chemical data
Formula
C17H23NO
Mol. mass
257.371 g/mol
Therapeutic considerations
Pregnancy cat.
?
Legal status
OTC (US)
(what is this?) (verify)
[3]
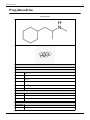
Dextrorphan (DXO) is a psychoactive drug of the morphinan chemical class which acts as an antitussive or cough
suppressant and dissociative hallucinogen. It is the dextro-stereoisomer of racemorphan, the levo-half being
levorphanol. Dextrorphan is produced by O-demethylation of dextromethorphan by CYP2D6. Dextrorphan is an
NMDA antagonist and contributes to the abuse liability of dextromethorphan.[4]
Dextrorphan
Pharmacology
•
•
•
•
Noncompetitive N-methyl-D-aspartate receptor (NMDAR) antagonist.[5] [6] [7]
σ1 and σ2 sigma receptor agonist.[8] [9]
α3β4, α4β2, and α7 nicotinic acetylcholine receptor antagonist.[10] [11]
L-type voltage-gated calcium channel (LVGCC) blocker.[7] [12]
The pharmacology of dextrorphan is similar to that of dextromethorphan. However, dextrorphan is much more
potent of an NMDA receptor antagonist and much weaker as a serotonin reuptake inhibitor in comparison.[13]
Therefore, it produces a noticeably more body-affecting, "stoned" feeling as opposed to the mental effects brought
about by DXM.
Legality
Dextrorphan was formerly a Schedule I controlled substance in the United States, but was unscheduled on October 1,
1976.[14]
References
[1] http:/ / www. nlm. nih. gov/ cgi/ mesh/ 2009/ MB_cgi?term=125-73-5& rn=1
[2] http:/ / pubchem. ncbi. nlm. nih. gov/ summary/ summary. cgi?cid=5360697
[3] http:/ / en. wikipedia. org/ w/ index. php?& diff=cur& oldid=408496712
[4] Zawertailo LA, Kaplan HL, Busto UE, Tyndale RF, Sellers EM (August 1998). "Psychotropic effects of dextromethorphan are altered by the
CYP2D6 polymorphism: a pilot study" (http:/ / www. ncbi. nlm. nih. gov/ pubmed/ 9690700). Journal of clinical psychopharmacology 18 (4):
332–7. PMID 9690700. .
[5] Wong BY, Coulter DA, Choi DW, Prince DA (February 1988). "Dextrorphan and dextromethorphan, common antitussives, are antiepileptic
and antagonize N-methyl-D-aspartate in brain slices". Neuroscience Letters 85 (2): 261–6. doi:10.1016/0304-3940(88)90362-X.
PMID 2897648.
[6] Church J, Jones MG, Davies SN, Lodge D (June 1989). "Antitussive agents as N-methylaspartate antagonists: further studies". Canadian
Journal of Physiology and Pharmacology 67 (6): 561–7. PMID 2673498.
[7] Kamel IR, Wendling WW, Chen D, Wendling KS, Harakal C, Carlsson C (October 2008). "N-methyl-D-aspartate (NMDA)
antagonists--S(+)-ketamine, dextrorphan, and dextromethorphan--act as calcium antagonists on bovine cerebral arteries" (http:/ / meta.
wkhealth. com/ pt/ pt-core/ template-journal/ lwwgateway/ media/ landingpage. htm?issn=0898-4921& volume=20& issue=4& spage=241).
Journal of Neurosurgical Anesthesiology 20 (4): 241–8. doi:10.1097/ANA.0b013e31817f523f. PMID 18812887. .
[8] Richter A, Löscher W (January 1997). "Dextrorphan, but not dextromethorphan, exerts weak antidystonic effects in mutant dystonic
hamsters" (http:/ / linkinghub. elsevier. com/ retrieve/ pii/ S0006-8993(96)01254-1). Brain Research 745 (1-2): 336–8.
doi:10.1016/S0006-8993(96)01254-1. PMID 9037429. .
[9] Chou YC, Liao JF, Chang WY, Lin MF, Chen CF (March 1999). "Binding of dimemorfan to sigma-1 receptor and its anticonvulsant and
locomotor effects in mice, compared with dextromethorphan and dextrorphan" (http:/ / linkinghub. elsevier. com/ retrieve/ pii/
S0006-8993(99)01125-7). Brain Research 821 (2): 516–9. doi:10.1016/S0006-8993(99)01125-7. PMID 10064839. .
[10] Damaj MI, Flood P, Ho KK, May EL, Martin BR (February 2005). "Effect of dextrometorphan and dextrorphan on nicotine and neuronal
nicotinic receptors: in vitro and in vivo selectivity" (http:/ / jpet. aspetjournals. org/ cgi/ pmidlookup?view=long& pmid=15356218). The
Journal of Pharmacology and Experimental Therapeutics 312 (2): 780–5. doi:10.1124/jpet.104.075093. PMID 15356218. .
[11] Hernandez SC, Bertolino M, Xiao Y, Pringle KE, Caruso FS, Kellar KJ (2000). "Dextromethorphan and its metabolite dextrorphan block
alpha3beta4 neuronal nicotinic receptors". J. Pharmacol. Exp. Ther. 293 (3): 962–7. PMID 10869398.
[12] Kim HC, Ko KH, Kim WK, et al. (May 2001). "Effects of dextromethorphan on the seizures induced by kainate and the calcium channel
agonist BAY k-8644: comparison with the effects of dextrorphan" (http:/ / linkinghub. elsevier. com/ retrieve/ pii/ S0166432800003727).
Behavioural Brain Research 120 (2): 169–75. doi:10.1016/S0166-4328(00)00372-7. PMID 11182165. .
[13] "Comparison of the Effects of Dextromethorphan, Dextrorphan, and Levorphanol on the Hypothalamo-Pituitary-Adrenal Axis -- Pechnick
and Poland 309 (2): 515 -- Journal of Pharmacology And Experimental Therapeutics" (http:/ / jpet. aspetjournals. org/ cgi/ content/ full/ 309/
2/ 515?ijkey=73c6efcf7713b02798c855b5ce4d50f1aa22d299& keytype2=tf_ipsecsha#ABS). .
[14] DEA. "Lists of: Scheduling Actions Controlled Substances Regulated Chemicals" (http:/ / www. deadiversion. usdoj. gov/ schedules/
orangebook/ orangebook. pdf). . Retrieved 2010-09-024.
22
Dopamine reuptake inhibitor
23
Dopamine reuptake inhibitor
A dopamine reuptake inhibitor (DRI, DARI) is a type of drug
that acts as a reuptake inhibitor for the neurotransmitter dopamine
by blocking the action of the dopamine transporter (DAT). This in
turn leads to increased extracellular concentrations of dopamine
and therefore an increase in dopaminergic neurotransmission.
Dopamine
Indications
DRIs may be used in the clinical treatment of attention-deficit hyperactivity disorder (ADHD), narcolepsy, and
fatigue or lethargy as stimulants, obesity as anorectics or appetite suppressants for weight loss purposes, as well as
mood disorders such as major depressive disorder (MDD) usually of the treatment-resistant or atypical variants as
antidepressants, social phobia (SP) also known as social anxiety disorder (SAD), and perhaps other anxiety disorders
as anxiolytics, parkinsonism such as that seen in Parkinson's disease as antiparkinsonian agents, for palliative care of
cancer related lethargy, drug addiction and/or dependence as anticraving agents, and both as augmentations and to
offset some of the side effects of other drugs like the selective serotonin reuptake inhibitors (SSRIs), such as sexual
dysfunction.
Effects
General
DRIs can induce a wide range of psychological and physiological effects, including the following:
Psychological
•
•
•
•
•
•
•
•
•
•
•
•
•
•
•
•
•
•
•
A general and subjective alteration in consciousness
Stimulation, arousal, and hyperactivity
Increased alertness, awareness, and wakefulness
Increased energy and endurance
Insomnia or inability to fall asleep
Agitation or restlessness
Enhanced attention, focus, and concentration
Increased desire, drive, and motivation
Improved cognition, memory, and learning
Goal-oriented thoughts or organized behavior
Rapid speech and/or racing thoughts
Antidepressant benefits or mood lift
Euphoria and/or rushes of pleasure
Anxiolysis and/or stress reduction
Sociability and/or talkativeness, as well as enhanced charisma and/or humor
Increased self-confidence, arrogance, and/or egotism
Feelings of power, grandiosity, and superiority
Irritability, aggression, anger and/or rage
Analgesia or pain relief
Dopamine reuptake inhibitor
• Impulsivity or impetuousness
• Hypersexuality and aphrodisiac effects
• Anorexia or decreased appetite and subsequent weight loss
Physiological
•
•
•
•
•
•
•
•
•
•
•
Dizziness, lightheadedness, or vertigo
Mydriasis or pupil dilation
Xerostomia or dry mouth
Nausea and/or emesis or vomiting
Gastrointestinal disturbances such as diarrhea and/or constipation
Headache or migraine
Trembling, shakiness, or muscle tremors
Hypertension or increased blood pressure
Tachycardia or increased heart rate
Hyperthermia or increased body temperature
Hyperhidrosis or increased perspiration or sweating
Miscellaneous
• Increased or decreased drug cravings and/or addiction (depending on the setting and usage)
• Drug tolerance with time and/or chronic administration, potentially resulting in dependence
• Drug interactions such as abolished effects from dopamine releasing agents like amphetamine
It should be noted, however, that many of these properties are dependent on whether the DRI in question is capable
of crossing the blood-brain-barrier (BBB). Those that do not will only produce peripheral effects.
Overdose
At very high doses and/or with chronic administration characterized by overdose, stimulant psychosis may develop,
the symptoms of which can include the following:
Psychological
• Disorientation and/or confusion
• Anxiety, severe paranoia, and/or panic attacks
• Hypervigilance or increased sensitivity to perceptual stimuli, accompanied by significantly increased threat
detection
• Hypomania or full-blown mania
• Derealization and/or depersonalization
• Hallucinations and/or delusions
• Thought disorder or disorganized thinking
• Cognitive and memory impairment potentially to the point of retrograde or anterograde amnesia
• Temporary psychosis
Physiological
•
•
•
•
•
Myoclonus or involuntary and intense muscle twitching
Hyperreflexia or overresponsive/overreactive reflexes
Syncope or fainting or loss of consciousness
Seizures or convulsions
Coma and/or death
Miscellaneous
• Neurotoxicity or brain damage
24
Dopamine reuptake inhibitor
Additionally, potential incarceration, hospitalization, institutionalization, and/or death, on account of extreme erratic
behavior which may include acts of crime, assault, accidental or intentional self-injury, and/or suicide, as well as
illicit drug abuse, may ensue under such circumstances.
Abuse
Due to their strong rewarding and reinforcing properties, DRIs are notorious for their high abuse potential and
liability to cause cravings, addiction, and dependence . DRIs such as cocaine, methylphenidate, and some tricyclic
antidepressants, combination releasing agents such as amphetamine, cocaine, methamphetamine, and MDMA
("ecstasy") are widely abused throughout the world. It is estimated that there are approximately six million people
addicted to cocaine in the United States (U.S.) alone.[1]
Notably, some DRIs have a lower abuse potential than others. Those that have a slow onset and long duration of
action such as bupropion (Wellbutrin, Zyban) are typically much less reinforcing than faster acting ones which
produce a rush like cocaine.[2] In fact, bupropion is often used as a maintenance therapy for treating stimulant
addiction.[3] However, depending on the route of administration (e.g., insufflation, inhalation, or injection), the
pleasurable effects of the DRI in question can be dramatically enhanced, potentially rendering those with only mild
rewarding effects to become far more reinforcing than they would be under normal circumstances.
List of DRIs
Pharmaceutical Drugs
•
•
•
•
•
•
•
•
•
•
•
•
•
•
•
•
•
•
•
•
•
•
•
Amineptine (Survector, Maneon, Directim)
Armodafinil (Nuvigil)
Benzatropine/Benztropine (Cogentin)[4]
Bupropion (Wellbutrin, Zyban)
Dexmethylphenidate (Focalin)
Dextroamphetamine (Dexedrine)
Esketamine (Ketanest S)[5]
Etybenzatropine/Ethybenztropine (Panolid, Ponalid, Ponalide)
Fencamfamine (Glucoenergan, Reactivan)
Fencamine (Altimina, Sicoclor)
Ketamine (Ketalar, Ketaset, Ketanest, Ketaject)[5]
Lefetamine (Santenol)
Medifoxamine (Cledial)
Mesocarb (Sidnocarb, Sydnocarb)
Methylphenidate (Ritalin, Concerta)
Modafinil (Provigil)
Nefopam (Acupan)
Nomifensine (Merital)
Pipradrol (Meretran)
Prolintane (Promotil, Katovit)
Pyrovalerone (Centroton, Thymergix)
Tiletamine (Telazol, Rompun)
Tripelennamine (Pyribenzamine)[6] [7] [8]
Street Drugs
• Cocaine (found in Erythroxylum coca (Coca))
• Desoxypipradrol (2-DPMP)
25
Dopamine reuptake inhibitor
•
•
•
•
•
•
Diphenylprolinol (D2PM)
Eticyclidine (PCE)
Methylenedioxypyrovalerone (MDPV)
Phencyclidine (PCP)[9]
Rolicyclidine (PCPy)
Tenocyclidine (TCP)
Research Chemicals
•
•
•
•
•
•
•
•
•
•
Altropane (IACFT; O-587)
Amfonelic Acid (AFA; WIN-25,978)
Benocyclidine (BTCP; GK-13)
Brasofensine (NS-2214)
Bromantane (ADK-709)
DBL-583
Dichloropane (RTI-111, O-401)
Diclofensine (Ro-8-4650)
Dieticyclidine
Difemetorex
•
•
•
•
•
•
•
•
•
•
•
•
Difluoropine (O-620)
Gacyclidine (GK-11)
GBR-12,935
Indatraline (Lu-19-005)
Ioflupane (β-CIT-FP)
Iometopane (β-CIT, RTI-55)
Manifaxine (GW-320,659
Radafaxine (GW-353,162)
Tametraline (CP-24,411)
Tesofensine (NS-2330)
Troparil (β-CPT; WIN-35,065-2)
Vanoxerine (GBR-12,909)
Natural Sources
• Hypericum perforatum (St. John's Wort)
• Chaenomeles Speciosa (Flowering Quince)[10]
• Psoralea Corylifolia (Babchi)[11]
Of the above listed agents, altropane, amfonelic acid, benocyclidine, DBL-583, difluoropine, GBR-12,935,
ioflupane, and vanoxerine are all highly selective, pure DRIs, with no known significant affinity for the serotonin or
norepinephrine transporters or any other sites.
Dopamine releasing agents (DRAs) such as amphetamine and methamphetamine also function as DRIs secondary to
their releasing action. To distinguish between DRIs and DRAs, the latter are not included in the above list. For a list
of DRAs, see the releasing agent article. In correspondence with the previous paragraph, notably, to date, there are
no known selective DRAs, as dissociating affinity between the dopamine and norepinephrine transporters has so far
proven to be virtually impossible to achieve, likely on account of the very similar structure of the respective
proteins.[12]
26
Dopamine reuptake inhibitor
References
[1] http:/ / www. drugabuse. gov/ STRC/ Forms. html#Cocaine
[2] Gardner EL, Liu X, Paredes W, et al. (October 2006). "A slow-onset, long-duration indanamine monoamine reuptake inhibitor as a potential
maintenance pharmacotherapy for psychostimulant abuse: effects in laboratory rat models relating to addiction". Neuropharmacology 51 (5):
993–1003. doi:10.1016/j.neuropharm.2006.06.009. PMID 16901516.
[3] Elkashef, A.M. 2005. Bupropion for the Treatment of Methamphetamine Dependence. Pages 1162–1170, in Neuropsychopharmacology
(2008) 33
[4] Schmitt KC, Zhen J, Kharkar P, et al. (November 2008). "Interaction of cocaine-, benztropine-, and GBR12909-like compounds with
wild-type and mutant human dopamine transporters: molecular features that differentially determine antagonist-binding properties". Journal of
Neurochemistry 107 (4): 928–40. doi:10.1111/j.1471-4159.2008.05667.x. PMC 2728472. PMID 18786172.
[5] Nishimura M, Sato K, Okada T, et al. (March 1998). "Ketamine inhibits monoamine transporters expressed in human embryonic kidney 293
cells". Anesthesiology 88 (3): 768–74. doi:10.1097/00000542-199803000-00029. PMID 9523822.
[6] Oishi R, Shishido S, Yamori M, Saeki K (February 1994). "Comparison of the effects of eleven histamine H1-receptor antagonists on
monoamine turnover in the mouse brain". Naunyn-Schmiedeberg's Archives of Pharmacology 349 (2): 140–4. PMID 7513381.
[7] Sato T, Suemaru K, Matsunaga K, Hamaoka S, Gomita Y, Oishi R (May 1996). "Potentiation of L-dopa-induced behavioral excitement by
histamine H1-receptor antagonists in mice". Japanese Journal of Pharmacology 71 (1): 81–4. doi:10.1254/jjp.71.81. PMID 8791174.
[8] Yeh SY, Dersch C, Rothman R, Cadet JL (September 1999). "Effects of antihistamines on 3, 4-methylenedioxymethamphetamine-induced
depletion of serotonin in rats". Synapse 33 (3): 207–17. doi:10.1002/(SICI)1098-2396(19990901)33:3<207::AID-SYN5>3.0.CO;2-8.
PMID 10420168.
[9] Pechnick RN, Bresee CJ, Poland RE (March 2006). "The role of antagonism of NMDA receptor-mediated neurotransmission and inhibition
of the dopamine reuptake in the neuroendocrine effects of phencyclidine". Life Sciences 78 (17): 2006–11. doi:10.1016/j.lfs.2005.09.018.
PMID 16288927.
[10] Zhao G, Jiang ZH, Zheng XW, Zang SY, Guo LH (September 2008). "Dopamine transporter inhibitory and antiparkinsonian effect of
common flowering quince extract". Pharmacology, Biochemistry, and Behavior 90 (3): 363–71. doi:10.1016/j.pbb.2008.03.014.
PMID 18485464.
[11] Zhao G, Li S, Qin GW, Fei J, Guo LH (July 2007). "Inhibitive effects of Fructus Psoraleae extract on dopamine transporter and
noradrenaline transporter". Journal of Ethnopharmacology 112 (3): 498–506. doi:10.1016/j.jep.2007.04.013. PMID 17555897.
[12] Rothman RB, Blough BE, Baumann MH (2007). "Dual dopamine/serotonin releasers as potential medications for stimulant and alcohol
addictions". The AAPS Journal 9 (1): E1–10. doi:10.1208/aapsj0901001. PMC 2751297. PMID 17408232.
27
FX-1006A
28
FX-1006A
Fx-1006A is an experimental drug for medical treatment of familial amyloid polyneuropathy.
Fx-1006A, (aka tafamidis[1] meglumine) has completed a phase II/III clinical trial[2] and preliminary results suggest
it slows progression of the disease.[3]
It has also completed a phase II clinical trial for Non-V30M transthyretin amyloidosis.[4]
References
[1] http:/ / portal. acs. org/ portal/ acs/ corg/ content?_nfpb=true& _pageLabel=PP_ARTICLEMAIN& node_id=841&
content_id=CNBP_024655& use_sec=true& sec_url_var=region1 Tafamidis structure
[2] http:/ / clinicaltrials. gov/ ct2/ show/ NCT00409175 Safety and Efficacy Study of Fx-1006A in Patients With Familial Amyloidosis.
[3] http:/ / protomag. ticsnetwork. com/ statics/ MGH_SP10_Protein_fold_F2. pdf
[4] http:/ / clinicaltrials. gov/ ct2/ show/ NCT00630864 The Effects of Fx-1006A on Transthyretin Stabilization and Clinical Outcome Measures
in Patients With Non-V30M Transthyretin Amyloidosis.
GABA agonist
A GABA agonist is a drug which acts to stimulate or increase the
action at the GABA receptor, producing typically sedative effects, and
may also cause other effects such as anxiolytic and muscle relaxant
effects.
Examples include:
Gamma-aminobutyric acid.
• Acamprosate
•
•
•
•
•
•
•
•
•
Picamilon
ethanol
GHB
benzodiazepines
nonbenzodiazepines (Zolpidem, Zopiclone, Zaleplon)
barbiturates
methaqualone
baclofen
muscimol
• progabide
• tiagabine
External links
• MeSH GABA+agonists [1]
• MeSH list of agents 82018755 [2]
References
[1] http:/ / www. nlm. nih. gov/ cgi/ mesh/ 2011/ MB_cgi?mode=& term=GABA+ agonists
[2] http:/ / www. ncbi. nlm. nih. gov/ sites/ entrez?Db=mesh& Cmd=ShowDetailView& TermToSearch=82018755
Melatonin
29
Melatonin
Melatonin
Systematic (IUPAC) name
N-[2-(5-methoxy-1H-indol-3-yl)ethyl]
ethanamide
Identifiers
[1]
CAS number
73-31-4
ATC code
N05 CH01
PubChem
CID 896
[2]
[3]
IUPHAR ligand 224 [4]
[5]
DrugBank
APRD00742
ChemSpider
872
UNII
JL5DK93RCL
KEGG
D08170
ChEMBL
CHEMBL45
[6]
[7]
[8]
[9]
Chemical data
Melatonin
30
Formula
C13H16N2O2
Mol. mass
232.278 g/mol
SMILES
eMolecules
[10]
& PubChem
[11]
Pharmacokinetic data
Bioavailability
30 – 50%
Metabolism
Hepatic via CYP1A2 mediated 6-hydroxylation
Half-life
35 to 50 minutes
Excretion
Urine
Therapeutic considerations
Pregnancy cat.
?
Legal status
Prescription Only (S4) (AU) ? (UK) OTC (US)
Routes
In humans: orally, as capsules, tablets or liquid, sublingually, or as transdermal patches. In lab animals: also
injection.
(what is this?) (verify)
[12]
Melatonin
(
/melatonin-pronunciation.oggˌmɛləˈtoʊnɪn/),
also
known
chemically
as
N-acetyl-5-methoxytryptamine,[13] is a naturally occurring compound found in animals, plants and microbes.[14]
[15]
In animals, circulating levels of the hormone melatonin vary in a daily cycle, thereby allowing the entrainment of
the circadian rhythms of several biological functions.[16]
Many biological effects of melatonin are produced through activation of melatonin receptors,[17] while others are due
to its role as a pervasive and powerful antioxidant,[18] with a particular role in the protection of nuclear and
mitochondrial DNA.[19]
In mammals, melatonin is secreted into the blood by the pineal gland in the brain. Known as the "hormone of
darkness" it is secreted in darkness in both day-active (diurnal) and night-active (nocturnal) animals.[20]
It may also be produced by a variety of peripheral cells such as bone marrow cells,[21] [22] lymphocytes and epithelial
cells. Usually, the melatonin concentration in these cells is much higher than that found in the blood but it does not
seem to be regulated by the photoperiod.
Melatonin-rich plant feed, such as rice, ingested by chicks has been shown to reach and bind to melatonin receptors
in their brains.[23] No food has been found to elevate plasma melatonin levels in humans.[24]
Products containing melatonin have been available over-the-counter as a dietary supplement in the United States
since the mid-1990s.[25] In many other countries, the over-the-counter sale of this neurohormone is not permitted or
requires a prescription, and the U.S. Postal Service lists unapproved melatonin preparations among items prohibited
by Germany.[26] In 2008, a prolonged release form of melatonin (Circadin®) by Lundbeck was approved in
European countries and Israel as a prescription drug for the treatment of insomnia.[27]
Melatonin
Medical uses
Melatonin has been studied for the treatment of cancer, immune disorders, cardiovascular diseases, depression,
seasonal affective disorder (SAD), circadian rhythm sleep disorders and sexual dysfunction. It may ameliorate
circadian misalignment and SAD.[28] Basic research indicates that melatonin may play a significant role in
modulating the effects of drugs of abuse such as cocaine.[29]
Circadian rhythm disorders
Exogenous melatonin taken in the evening is, together with light therapy upon awakening, the standard treatment for
delayed sleep phase syndrome (DSPS) and non-24-hour sleep-wake syndrome. It appears to have some use against
other circadian rhythm sleep disorders as well, such as jet lag and the problems of people who work rotating or night
shifts. Melatonin reduces sleep onset latency to a greater extent in people with DSPS than in people with
insomnia.[30]
Taken 30 to 90 minutes before bedtime, melatonin supplementation acts as a mild hypnotic. It causes melatonin
levels in the blood to rise earlier than the brain's own production accomplishes. This usage is now common in sleep
and relaxation drinks.[31]
A very small dose taken several hours before bedtime in accordance with the phase response curve for melatonin in
humans (PRC) doesn't cause sleepiness but, acting as a chronobiotic (affecting aspects of biological time
structure),[32] advances the phase slightly and is additive to the effect of using light therapy upon awakening. Light
therapy may advance the phase about one to two-and-a-half hours and a small oral dose of melatonin, timed correctly
some hours before bedtime, can add about 30 minutes to the advance achieved with light therapy.[33]
Studies have shown that late night shift work may be considered a cancer-causing agent. Melatonin is an anti-oxidant
and suppressant of tumor development that is produced at night; when someone works in artificial light, they
generally have lower melatonin and may be more likely to develop cancer. Melatonin supplements are useful in that
they may simulate the Melatonin production at different times that does not occur during regular sleeping hours for
people who work night shifts.[34]
Learning, memory and Alzheimer's
Melatonin receptors appear to be important in mechanisms of learning and memory in mice,[35] and melatonin can
alter electrophysiological processes associated with memory, such as long-term potentiation (LTP). The first
published evidence that melatonin may be useful in Alzheimer's disease was the demonstration that this
neurohormone prevents neuronal death caused by exposure to the amyloid beta protein, a neurotoxic substance that
accumulates in the brains of patients with the disorder.[36] Melatonin also inhibits the aggregation of the amyloid
beta protein into neurotoxic microaggregates that, it seems, underlie the neurotoxicity of this protein, causing death
of neurons and formation of neurofibrillary tangles, the other neuropathological landmark of Alzheimer's disease.[37]
Melatonin has been shown to prevent the hyperphosphorylation of the tau protein in rats. Hyperphosphorylation of
tau protein can also result in the formation of neurofibrillary tangles. Studies in rats suggest that melatonin may be
effective for treating Alzheimer's disease.[38] These same neurofibrillary tangles can be found in the hypothalamus in
patients with Alzheimer's, adversely affecting their bodies' production of melatonin. Those Alzheimer's patients with
this specific affliction often show heightened afternoon agitation, called sundowning, which has been shown in many
studies to be effectively treated with melatonin supplements in the evening.[39]
31
Melatonin
Delirium
A randomized placebo-controlled trial, showed that low-dose (0.5 mg) melatonin supplementation to elderly patients
admitted to acute Medicine services, significantly reduced delirium.[40]
ADHD
Research shows that after melatonin is administered to ADHD patients on methylphenidate, the time needed to fall
asleep is significantly reduced. Furthermore, the effects of the melatonin after three months showed no change from
its effects after one week of use.[41]
Fertility
A research team in Italy has found that melatonin supplementation in the evening in perimenopausal women
produces an improvement in thyroid function and gonadotropin levels, as well as restoring fertility and menstruation
and preventing the depression associated with the menopause.[42] However, at the same time, some resources warn
women trying to conceive not to take a melatonin supplement.[43] One study reported that three mg of melatonin
taken in the evening raised prolactin levels in six out of seven women.[44] Melatonin also lowers FSH levels. It is
believed that these hormonal changes could in some women impair fertility.[45]
Toxicology
Melatonin has a very low toxicity in rats. Rat maternal toxicity: The no observable adverse effect level (NOAEL)
and lowest observed adverse effect level (LOAEL) were 100 and 200 mg/kg/day, respectively, and the
developmental toxicity NOAEL was >= 200 mg/kg/day.[46]
Headaches
Several clinical studies indicate that supplementation with melatonin is an effective preventive treatment for
migraines and cluster headaches.[47] [48]
Mood disorders
Melatonin has been shown to be effective in treating one form of depression and seasonal affective disorder,[49] and
is being considered for bipolar and other disorders in which circadian disturbances are involved.[50] It has been
observed that bipolar disorder might have, as a "trait marker" (something that is characteristic of being bipolar, that
does not change with state), supersensitivity to light, i.e., a greater decrease in melatonin secretion in response to
light exposure at night.[51] This could be contrasted with drug-free recovered bipolar patients not showing light
hypersensitivity.[52]
Cancer
A systematic review of unblinded clinical trials involving a total of 643 cancer patients using melatonin found a
reduced incidence of death.[53] Another clinical trial is due to be completed in 2012.[54] Melatonin levels at night are
reduced to 50% by exposure to a low-level incandescent bulb for only 39 minutes, and it has been suspected that
women with the brightest bedrooms have an increased risk for breast cancer.[55] Reduced melatonin production has
been proposed as a likely factor in the significantly higher cancer rates in night workers.[56]
32
Melatonin
Gallbladder stones
Melatonin presence in the gallbladder has many protective properties, such as converting cholesterol to bile,
preventing oxidative stress, and increasing the mobility of gallstones from the gallbladder.[57] It also decreases the
amount of cholesterol produced in the gallbladder by regulating the cholesterol that passes through the intestinal
wall. In guinea pigs, melatonin administration restored normal function by reducing inflammation after induced
Cholecystitis, whether administered before or after onset of inflammation.[57] Concentration of melatonin in the bile
is 2–3 times higher than the otherwise very low daytime melatonin levels in the blood across many diurnal
mammals, including humans.[58]
Amyotrophic lateral sclerosis
In animal models, melatonin has been shown to ameliorate glutamate-induced neuronal death, it is presumed due to
its antioxidant effects. In a clinical safety study involving 31 ALS patients, high-dose rectal melatonin (300 mg/day
for 2 years) was shown to be tolerated well.[59]
Obesity
Melatonin is involved in energy metabolism and body weight control in small animals. Many studies show that
chronic melatonin supplementation in drinking water reduces body weight and abdominal fat in experimental
animals, especially in the middle-aged rats.[60] It is interesting to note that the weight loss effect of melatonin does
not require the animals to eat less and to be physically more active. A potential mechanism is that melatonin
promotes the recruitment of brown adipose tissue (BAT) as well as enhances its activity.[61] This effect would raise
the basal metabolic rate by stimulating thermogenesis, heat generation through uncoupling oxidative phosphorylation
in mitochondria. Whether the results of animal studies can be extrapolated to human obesity is a matter of future
clinical trials, since substantially active BAT has been identified in adult humans.
Protection from radiation
Radioactive fallout from nuclear accidents is potentially a worldwide problem and we are ill-equipped to protect
against this low dose and persistent ionizing radiation, for example, after cesium-137 ingestion or inhalation. Both
animal [62] and human[63] [64] studies have shown melatonin to be potentially radioprotective. Moreover, it is a more
efficient protector than amifostine,[65] a commonly used agent for this purpose. The mechanism of melatonin in
protection against ionizing radiation is thought to involve scavenging of free radicals.[66] It is estimated that nearly
70% of biological damage caused by ionizing radiation is attributable to the free radical, especially the hydroxyl
radical that attacks DNA, proteins, and cellular membranes. Melatonin has been suggested as a radioprotective agent,
with the proposed advantages of being broadly protective, readily available, orally self-administered, and without
major known side effects.[67]
Other
It is believed that melatonin has some effects for sexual development in higher organisms.,[68] and is involved in the
seasonal timing of reproduction in rodents.
Melatonin increases proliferation of cultured neural stem cells obtained from mice nervous tissue.[69]
Melatonin was used in to treat Periodic Limb Movement Disorder, a common neurological condition, which, when
severe, adversely affects sleep and causes excessive daytime fatigue, in a small trial conducted by Kunz D and Bes
F. In this condition, the sufferer is affected by mini arousals during sleep and limb movements that occur in a
frequent rhythmic fashion. This often involves leg kicking, but sometimes also involves arm movement. Those
affected are often not aware of the condition, and partners are often the first to notice the condition. 7 out of the 9
participants in the trial showed significant improvement.[70]
33
Melatonin
In recent trial for use in IBS treatment, melatonin relieved some symptoms, as published in 2010[71]
Adverse effects
Melatonin appears to cause very few side-effects in the short term, up to three months, when healthy people take it at
low doses. A systematic review[72] in 2006 looked specifically at efficacy and safety in two categories of melatonin
usage: first, for sleep disturbances that are secondary to other diagnoses and, second, for sleep disorders such as jet
lag and shift work that accompany sleep restriction.
The study concluded that There is evidence that melatonin is safe with short term use.
A similar analysis[73] by the same team a year earlier on the efficacy and safety of exogenous melatonin in the
management of primary sleep disorders found that: There is evidence to suggest that melatonin is safe with
short-term use (3 months or less).
Some unwanted effects in some people, especially at high doses (~3 mg/day or more) may include: headaches,
nausea, next-day grogginess or irritability, hormone fluctuations, vivid dreams or nightmares,[74] reduced blood
flow, and Hypothermia [75]
While no large, long-term studies that might reveal side-effects have been conducted, there do exist case reports
about patients having taken melatonin for years.[76]
Melatonin can cause somnolence (drowsiness), and, therefore, caution should be shown when driving, operating
machinery, etc.
In individuals with auto-immune disorders, there is concern that melatonin supplementation may ameliorate or
exacerbate symptoms due to immunomodulation.[77] [78]
Individuals experiencing orthostatic intolerance, a cardiovascular condition that results in reduced blood pressure
and blood flow to the brain when a person stands, may experience a worsening of symptoms when taking melatonin
supplements, a study at Penn State College of Medicine's Milton S. Hershey Medical Center suggests. Melatonin can
exacerbate symptoms by reducing nerve activity in those experiencing the condition, the study found.[79]
The use of melatonin derived from animal pineal tissue may carry the risk of contamination or the means of
transmitting viral material. The synthetic form of this medication does not carry this risk.[16] [80]
In plants
Melatonin has been identified in many plants.[15] It occurs in trace amounts in some foods, especially cherries to
about 0.17-13.46 ng/g.[81] The physiological roles of melatonin in plants involve regulation of their response to
photoperiod, defense against harsh environments, and the function of an antioxidant. The latter may be the original
function of melatonin in organisms with the others being added during evolution.[82] Melatonin has been reported in
foodstuffs including bananas and grapes, rice and cereals, herbs, olive oil, wine and beer. While no food has been
found to elevate plasma melatonin levels in humans,[24] when other animals consume melatonin-containing food,
blood levels of melatonin do increase.[23]
In animals
Many animals use the variation in duration of melatonin production each day as a seasonal clock.[83] In animals
including humans[84] the profile of melatonin synthesis and secretion is affected by the variable duration of night in
summer as compared to winter. The change in duration of secretion thus serves as a biological signal for the
organisation of daylength-dependent (photoperiodic) seasonal functions such as reproduction, behaviour, coat
growth and camouflage colouring in seasonal animals.[84] In seasonal breeders that do not have long gestation
periods and that mate during longer daylight hours, the melatonin signal controls the seasonal variation in their
sexual physiology, and similar physiological effects can be induced by exogenous melatonin in animals including
34
Melatonin
mynah birds[85] and hamsters.[86]
In mammals
Melatonin is produced in the pineal gland, which is outside of the blood-brain barrier, acts as an endocrine hormone
since it is released into the blood. By contrast, melatonin produced by the retina and the gastrointestinal (GI) tract
acts as a paracrine hormone.
Melatonin can suppress libido by inhibiting secretion of luteinizing hormone (LH) and follicle-stimulating hormone
(FSH) from the anterior pituitary gland, especially in mammals that have a breeding season when daylight hours are
long. The reproduction of long-day breeders is repressed by melatonin and the reproduction of short-day breeders is
stimulated by melatonin. During the night, melatonin regulates leptin, lowering the levels; see leptin.
Light/dark information reaches the suprachiasmatic nuclei (SCN) via retinal photosensitive ganglion cells,
intrinsically photosensitive photoreceptor cells, distinct from those involved in image forming (that is, these
light-sensitive cells are a third type in the retina, in addition to rods and cones). These cells represent approximately
2% of the retinal ganglion cells in humans and express the photopigment melanopsin.[87] The sensitivity of
melanopsin is consistent with that of a vitamin A-based photopigment, with a peak sensitivity at 484 nm (blue
light).[88] This photoperiod cue entrains the circadian rhythm, and the resultant production of specific "dark"- and
"light"-induced neural and endocrine signals that regulate behavioral and physiological circadian rhythms. Melatonin
is secreted in darkness in both day-active (diurnal) and night-active (nocturnal) animals.[20]
In humans
Circadian rhythm
In humans, melatonin is produced by the pineal gland, a gland about the size of a pea, located in the center of the
brain but outside the blood-brain barrier. The melatonin signal forms part of the system that regulates the sleep-wake
cycle by chemically causing drowsiness and lowering the body temperature, but it is the central nervous system (to
be more specific, the SCN) that controls the daily cycle in most components of the paracrine and endocrine
systems[89] [90] rather than the melatonin signal (as was once postulated).
Infants' melatonin levels become regular in about the third month after birth, with the highest levels measured
between midnight and 08:00 (8 AM).[91]
In humans, 90% of melatonin is cleared in a single passage through the liver, a small amount is excreted in urine,[30]
and a small amount is found in saliva.
Human melatonin production decreases as a person ages.[92] It is believed that melatonin levels are reduced in
teenagers as compared to other age groups, leading to later sleeping and waking times.[93]
Light dependence
Production of melatonin by the pineal gland is inhibited by light and permitted by darkness. For this reason
melatonin has been called "the hormone of darkness." Its onset each evening is called the Dim-Light Melatonin
Onset (DLMO). Secretion of melatonin as well as its level in the blood, peaks in the middle of the night, and
gradually falls during the second half of the night, with normal variations in timing according to an individual's
chronotype.[94] Terman et al. devised a formulation that mimics that gradual washout (vs. the spikes in blood
concentration and rapid washout associated with most over-the-counter melatonin tablets). When used several hours
before sleep, the compound shifts the circadian clock earlier, thus promoting earlier sleep onset and morning
awakening.[95]
It is principally blue light, around 460 to 480nm, that suppresses melatonin,[96] increasingly with increased light
intensity and length of exposure. Until recent history, humans in temperate climates were exposed to few hours of
35
Melatonin
(blue) daylight in the winter; their fires gave predominantly yellow light. Wearing glasses that block blue light in the
hours before bedtime may avoid melatonin loss. Kayumov et al. showed that light containing only wavelengths
greater than 530 nm does not suppress melatonin in bright-light conditions.[97] Use of blue-blocking goggles the last
hours before bedtime has also been advised for people who need to adjust to an earlier bedtime, as melatonin
promotes sleepiness.
Antioxidant
Besides its function as synchronizer of the biological clock, melatonin also exerts a powerful antioxidant activity.
The discovery of melatonin as an antioxidant was made in 1993.[98] In many less complex life forms, this is its only
known function.[99] Melatonin is an antioxidant that can easily cross cell membranes and the blood-brain barrier.[18]
Melatonin is a direct scavenger of OH, O2−, and NO.[100] Unlike other antioxidants, melatonin does not undergo
redox cycling, the ability of a molecule to undergo reduction and oxidation repeatedly. Redox cycling may allow
other antioxidants (such as vitamin C) to act as pro-oxidants, counterintuitively promoting free radical formation.
Melatonin, on the other hand, once oxidized, cannot be reduced to its former state because it forms several stable
end-products upon reacting with free radicals. Therefore, it has been referred to as a terminal (or suicidal)
antioxidant.[101]
Recent research indicates that the first metabolite of melatonin in the melatonin antioxidant pathway may be
N(1)-acetyl-N(2)-formyl-5-methoxykynuramine (or AFMK) rather than the common, excreted 6-hydroxymelatonin
sulfate. AFMK alone is detectable in unicellular organisms and metazoans. A single AFMK molecule can neutralize
up to 10 ROS/RNS (reactive oxygen species/reactive nitrogen species) since many of the products of the
reaction/derivatives (including melatonin) are themselves antioxidants. This capacity to absorb free radicals extends
at least to the quaternary metabolites of melatonin, a process referred to as "the free radical scavenging cascade."
This is not true of other, conventional antioxidants.[99]
In animal models, melatonin has been demonstrated to prevent the damage to DNA by some carcinogens, stopping
the mechanism by which they cause cancer.[102] It also has been found to be effective in protecting against brain
injury caused by ROS release in experimental hypoxic brain damage in newborn rats.[103] Melatonin's antioxidant
activity may reduce damage caused by some types of Parkinson's disease, may play a role in preventing cardiac
arrhythmia and may increase longevity; it has been shown to increase the average life span of mice by 20% in some
studies.[104] [105] [106]
Immune system
While it is known that melatonin interacts with the immune system,[107] [108] the details of those interactions are
unclear. There have been few trials designed to judge the effectiveness of melatonin in disease treatment. Most
existing data are based on small, incomplete clinical trials. Any positive immunological effect is thought to result
from melatonin acting on high affinity receptors (MT1 and MT2) expressed in immunocompetent cells. In
preclinical studies, melatonin may enhance cytokine production,[109] and by doing this counteract acquired
immunodeficiences. Some studies also suggest that melatonin might be useful fighting infectious disease[110]
including viral, such as HIV, and bacterial infections, and potentially in the treatment of cancer.[111]
Endogenous melatonin in human lymphocytes has been related to interleukin-2 (IL-2) production and to the
expression of IL-2 receptor.[112] This suggests that melatonin is involved in the clonal expansion of
antigen-stimulated human T lymphocytes. When taken in conjunction with calcium, it is an immunostimulator and is
used as an adjuvant in some clinical protocols; conversely, the increased immune system activity may aggravate
autoimmune disorders. In rheumatoid arthritis patients, melatonin production has been found increased when
compared to age-matched healthy controls.[113]
Although it has not yet been clearly demonstrated whether melatonin increases non-specific immunity with resulting
contraindication in autoimmune diseases, an increase in the production of IL-2 and IL-1 was noted in cultured
36
Melatonin
splenocytes.[114]
Dreaming
Some supplemental melatonin users report an increase in vivid dreaming. Extremely high doses of melatonin
(50 mg) dramatically increased REM sleep time and dream activity in both people with and without narcolepsy.[115]
Many psychoactive drugs, such as cannabis and lysergic acid diethylamide (LSD), increase melatonin synthesis.[115]
It has been suggested that nonpolar (lipid-soluble) indolic hallucinogenic drugs emulate melatonin activity in the
awakened state and that both act on the same areas of the brain.[115]
Autism
Individuals with autism spectrum disorders (ASD) may have lower than normal levels of melatonin. A 2008 study
found that unaffected parents of individuals with ASD also have lower melatonin levels, and that the deficits were
associated with low activity of the ASMT gene, which encodes the last enzyme of melatonin synthesis.[116]
Multiple small studies have demonstrated that 2 to 10 mg of melatonin may benefit children with ASD who have
trouble falling asleep and/or maintaining sleep. A small 2011 randomized crossover trial found that the
administration of melatonin, when compared to placebo, decreased sleep latency and increased total sleep time, but
had no effect on the number of night time awakenings.[117] At this time, no guidelines exist for the use of melatonin
in children with ASD.
Aging
Research has supported the anti-aging properties of melatonin. Younger children hit their peak melatonin production
at night, and some researchers believe that the level of melatonin peaks earlier as we get older. This may explain
why older adults go to bed earlier, wake up earlier, and have more sleep problems than children do.[118]
Some studies have shown that melatonin plays a crucial part in the aging process and that it may act as an anti-aging
agent when taken by older adults. It has been reported in one study that while elderly people have different gene
expression levels in 100 of 10,000 genes, administration of melatonin may reverse this change in gene expression
thus making the genes of elderly people similar to those of younger people.[119]
One study conducted by researchers of the University of Granada’s Institute of Biotechnology found that consuming
melatonin may neutralize oxidative damage and delay the neurodegenerative process of aging. When small amounts
of melatonin were administered to lab mice, it reduced the oxidative damage caused by aging and delayed the
inflammatory process, which in turn increased the longevity of the mice. The researchers hope these results can also
be applied to humans.[120]
37
Melatonin
Use as medication
The hormone melatonin is used to treat circadian rhythm sleep disorders and
some types of insomnia.
Studies have found that the use of melatonin can help entrain the circadian
clock to environmental cycles and have beneficial effects for the treatment of
certain forms of insomnia (2004).[121] Prolonged release melatonin has shown
good results in treating insomnia in older adults (2007).[122]
A 2004 review found that melatonin significantly increased total sleep time in
people suffering from sleep restriction.[30] Other studies have found that for
certain types of sleep disorders, melatonin is not effective. A 2006 review
found that although it is safe for short term use (of three months or less), there
is "no evidence that melatonin is effective in treating secondary sleep
disorders or sleep disorders accompanying sleep restriction, such as jet lag
and shiftwork disorder."[72]
In a 2005 study, researchers concluded that while "there is some evidence to
A bottle of melatonin tablets
suggest that melatonin is effective in treating delayed sleep phase syndrome,
...there is evidence to suggest that melatonin is not effective in treating most primary sleep disorders with short-term
use (4 weeks or less)."[73]
Dosage
Melatonin tablets/capsules often contain three to ten times the amount needed to produce physiologic nocturnal
blood melatonin levels for a more rapid sleep onset. Studies suggest that smaller doses (for example 0.3 mg as
opposed to 3 mg) are just as effective.[123]
Large doses of melatonin can even be counterproductive: Lewy et al.[124] provide support to the "idea that too much
melatonin may spill over onto the wrong zone of the melatonin phase-response curve" (PRC). In one of their blind
subjects, 0.5 mg of melatonin was effective while 20 mg was not. Solomon Labs tested initial doses of 30 and 60
milligrams and found very little efficacy even at those levels.[124] [125]
History
Melatonin is related to the mechanism by which some amphibians and reptiles change the color of their skin and,
indeed, it was in this connection the substance first was discovered.[126] [127] As early as 1917, McCord and Allen
discovered (J Exptl Zool, 1917) that extract of the pineal glands of cows lightened frog skin.[24] Dermatology
professor Aaron B. Lerner and colleagues at Yale University, in the hope that a substance from the pineal might be
useful in treating skin diseases, isolated and named the hormone melatonin in 1958.[128] In the mid-70s Lynch et al.
demonstrated[129] that the production of melatonin exhibits a circadian rhythm in human pineal glands. The
discovery that melatonin is an antioxidant was made in 1993.[98] Around the same time, the hormone got a lot of
press as a possible treatment for many illnesses.[130] The New England Journal of Medicine editorialized in 2000:
"The hype and the claims of the so-called miraculous powers of melatonin several years ago did a great disservice to
a scientific field of real importance to human health. (...) Our 24-hour society, with its chaotic time cues and lack of
natural light, may yet reap substantial benefits."[131]
38
Melatonin
Availability
Legal availability of melatonin varies in different countries, ranging from being available without prescription (e.g.,
in most of North America) to being available only on prescription or not at all (although its possession and use may
not be illegal). The hormone may be administered orally, as capsules, tablets or liquid, sublingually, or as
transdermal patches.
Dietary supplement
In the USA, because it is sold as a dietary supplement, sometimes combined with other ingredients, such as vitamins
and herbal extracts, and not as a drug, the Food and Drug Administration (FDA) regulations that apply to
medications are not applicable to melatonin.[16] However, new FDA rules required that by June 2010 all production
of dietary supplements must comply with "current good manufacturing practices" (cGMP), and be manufactured
with "controls that result in a consistent product free of contamination, with accurate labeling."[132] In addition, the
industry has been required to report to the FDA "all serious dietary supplement related adverse events" and the FDA
has, within the cGMP guidelines, recently begun enforcement of that requirement.
Pediatrics
While the packaging of melatonin often warns against use in children, at least one long-term study[133] does assess
effectiveness and safety in children. No serious safety concerns were noted in any of the 94 cases studied by means
of a structured questionnaire for the parents. With a mean follow-up time of 3.7 years, long-term medication was
effective against sleep onset problems in 88% of the cases.
Prolonged release
Melatonin is available as a prolonged-release prescription drug, trade-name Circadin, manufactured by Neurim
Pharmaceuticals. The European Medicines Agency (EMA) has approved Circadin 2 mg (prolonged-release
melatonin) for patients aged 55 or over, as monotherapy for the short-term treatment (up to 13 weeks) of primary
insomnia characterized by poor quality of sleep.[134]
References
[1] http:/ / www. nlm. nih. gov/ cgi/ mesh/ 2009/ MB_cgi?term=73-31-4& rn=1
[2] http:/ / www. whocc. no/ atc_ddd_index/ ?code=N05CH01
[3] http:/ / pubchem. ncbi. nlm. nih. gov/ summary/ summary. cgi?cid=896
[4] http:/ / www. iuphar-db. org/ DATABASE/ LigandDisplayForward?ligandId=224
[5] http:/ / www. drugbank. ca/ drugs/ APRD00742
[6] http:/ / www. chemspider. com/ Chemical-Structure. 872
[7] http:/ / fdasis. nlm. nih. gov/ srs/ srsdirect. jsp?regno=JL5DK93RCL
[8] http:/ / www. kegg. jp/ entry/ D08170
[9] https:/ / www. ebi. ac. uk/ chembldb/ index. php/ compound/ inspect/ CHEMBL45
[10] http:/ / www. emolecules. com/ cgi-bin/ search?t=ex& q=CC%28%3DO%29NCCc1c%5BnH%5Dc2c1cc%28cc2%29OC
[11] http:/ / pubchem. ncbi. nlm. nih. gov/ search/ ?smarts=CC%28%3DO%29NCCc1c%5BnH%5Dc2c1cc%28cc2%29OC
[12] http:/ / en. wikipedia. org/ w/ index. php?& diff=cur& oldid=420231802
[13] http:/ / www. sleepdex. org/ melatonin. htm
[14] Caniato R, Filippini R, Piovan A, Puricelli L, Borsarini A, Cappelletti EM (2003). "Melatonin in plants". Advances in Experimental
Medicine and Biology 527: 593–7. PMID 15206778.
[15] Paredes SD, Korkmaz A, Manchester LC, Tan DX, Reiter RJ (2009). "Phytomelatonin: a review". Journal of Experimental Botany 60 (1):
57–69. doi:10.1093/jxb/ern284. PMID 19033551.
[16] Altun A, Ugur-Altun B (2007). "Melatonin: therapeutic and clinical utilization". Int. J. Clin. Pract. 61 (5): 835–45.
doi:10.1111/j.1742-1241.2006.01191.x. PMID 17298593.
[17] Boutin JA, Audinot V, Ferry G, Delagrange P (August 2005). "Molecular tools to study melatonin pathways and actions". Trends in
Pharmacological Sciences 26 (8): 412–9. doi:10.1016/j.tips.2005.06.006. PMID 15992934.
39
Melatonin
[18] Hardeland R (July 2005). "Antioxidative protection by melatonin: multiplicity of mechanisms from radical detoxification to radical
avoidance". Endocrine 27 (2): 119–30. doi:10.1385/ENDO:27:2:119. PMID 16217125.
[19] Reiter RJ, Acuña-Castroviejo D, Tan DX, Burkhardt S (June 2001). "Free radical-mediated molecular damage. Mechanisms for the
protective actions of melatonin in the central nervous system". Annals of the New York Academy of Sciences 939 (1): 200–15.
doi:10.1111/j.1749-6632.2001.tb03627.x. PMID 11462772.
[20] Challet E (December 2007). "Minireview: Entrainment of the suprachiasmatic clockwork in diurnal and nocturnal mammals".
Endocrinology 148 (12): 5648–55. doi:10.1210/en.2007-0804. PMID 17901231.
[21] Maestroni GJ (March 2001). "The immunotherapeutic potential of melatonin". Expert Opin Investig Drugs 10 (3): 467–76.
doi:10.1517/13543784.10.3.467. PMID 11227046.
[22] Conti A, Conconi S, Hertens E, Skwarlo-Sonta K, Markowska M, Maestroni JM (May 2000). "Evidence for melatonin synthesis in mouse
and human bone marrow cells" (http:/ / www. blackwell-synergy. com/ openurl?genre=article& sid=nlm:pubmed& issn=0742-3098&
date=2000& volume=28& issue=4& spage=193). J. Pineal Res. 28 (4): 193–202. doi:10.1034/j.1600-079X.2000.280401.x. PMID 10831154.
.
[23] Hattori A, Migitaka H, Iigo M, et al. (March 1995). "Identification of melatonin in plants and its effects on plasma melatonin levels and
binding to melatonin receptors in vertebrates". Biochemistry and Molecular Biology International 35 (3): 627–34. PMID 7773197.
[24] Coates, Paul M. (2005). Encyclopedia of Dietary Supplements (http:/ / books. google. com/ ?id=Sfmc-fRCj10C& pg=PA457& lpg=PA457&
dq=Lerner+ melatonin+ history). Marc R. Blackman, Gordon M. Cragg, Mark Levine, Joel Moss, Jeffrey D. White. CRC Press. pp. 457–466.
ISBN 0824755049. . Retrieved 2009-03-31.
[25] Ratzburg, Courtney (Undated). "Melatonin: The Myths and Facts" (http:/ / www. vanderbilt. edu/ AnS/ psychology/ health_psychology/
melatonin. htm). Vanderbilt University. . Retrieved 2007-12-02.
[26] USPS. "Country Conditions for Mailing — Germany" (http:/ / pe. usps. gov/ text/ imm/ fh_011. htm). . Retrieved 2008-01-15.
[27] Circadin®, Retardtabletten, 2 mg (melatoninum) (http:/ / www. swissmedic. ch/ zulassungen/ 00171/ 00181/ 01154/ index. html?lang=de)
[28] Lewy A, Sack R, Miller L, Hoban T (1987). "Antidepressant and circadian phase-shifting effects of light". Science 235 (4786): 352–4.
doi:10.1126/science.3798117. PMID 3798117.
[29] Uz T, Akhisaroglu M, Ahmed R, Manev H (2003). "The pineal gland is critical for circadian Period1 expression in the striatum and for
circadian cocaine sensitization in mice". Neuropsychopharmacology 28 (12): 2117–23. doi:10.1038/sj.npp.1300254. PMID 12865893.
[30] Buscemi, N. et al. (2004). "Melatonin for Treatment of Sleep Disorders. Summary, Evidence Report/Technology Assessment: Number 108"
(http:/ / www. ahrq. gov/ clinic/ epcsums/ melatsum. htm) (Review). U.S. Department of Health & Human Services, Agency for Healthcare
Research and Quality. . Retrieved 2010-05-25.
[31] Weichselbaum, Simone (2010-02-07). "New melatonin-based drinks help restless sleepers hit the hay with just a gulp - but pack hormones"
(http:/ / www. nydailynews. com/ lifestyle/ 2010/ 02/ 07/
2010-02-07_these_drinksll_knock_you_out_dozeinducing_concoctions_include_dose_of_hormones. html). Daily News (New York). .
[32] Chronobiotics: Selected Agents of Potential Value in Jet Lag and other Dyschronisms, H. W. Simpson, of Glasgow University, in
Chronobiology: Principles and Application to Shifts in Schedules, ed: L.E. Sheving and F. Hagberg, Springer, Berlin, 1979 (http:/ / books.
google. co. uk/ books?id=e-bZhuzXbK4C& pg=PA433& lpg=PA433& dq=chronobiotic+ definition& source=bl& ots=boAbbe_1-r&
sig=H0ICqtUEACE9FK6d6dwiAIHF-Mw& hl=en& ei=bkO6SpuEJ5PajQfmo_n3BQ& sa=X& oi=book_result& ct=result&
resnum=7#v=onepage& q=chronobiotic definition& f=false). Retrieved September 23, 2009.
[33] Mundey K, Benloucif S, Harsanyi K, Dubocovich ML, Zee PC (October 2005). "Phase-dependent treatment of delayed sleep phase
syndrome with melatonin". Sleep 28 (10): 1271–8. PMID 16295212.
[34] . Straif, K., Baan, R., Grosse, Y., et al."Carcinogenicity of shift-work, painting, and fire-fighting." Lancet Oncology 8.12 (2007): 1065 1066.
[35] Larson J, Jessen R, Uz T, Arslan A, Kurtuncu M, Imbesi M, Manev H (2006). "Impaired hippocampal long-term potentiation in melatonin
MT2 receptor-deficient mice". Neurosci Lett 393 (1): 23–6. doi:10.1016/j.neulet.2005.09.040. PMID 16203090.
[36] Pappolla MA, Sos M, Omar RA, Bick RJ, Hickson-Bick DL, Reiter RJ, Efthimiopoulos S, Robakis NK. (1997). "Melatonin prevents death
of neuroblastoma cells exposed to the Alzheimer amyloid peptide". J Neurosci 17 (5): 1683–1690. PMID 9030627.
[37] Pappolla M, Bozner P, Soto C, Shao H, Robakis NK, Zagorski M, Frangione B, Ghiso J. (1998). "Inhibition of Alzheimer
beta-fibrillogenesis by melatonin". J Biol Chem 273 (13): 7185–7188. doi:10.1074/jbc.273.13.7185. PMID 9516407.
[38] Wang X, Zhang J, Yu X, Han L, Zhou Z, Zhang Y, Wang J (2005). "Prevention of isoproterenol-induced tau hyperphosphorylation by
melatonin in the rat". Sheng Li Xue Bao 57 (1): 7–12. PMID 15719129.
[39] Volicer L, Harper D, Manning B, Goldstein R, Satlin A (2001). "Sundowning and circadian rhythms in Alzheimer's disease". Am J
Psychiatry 158 (5): 704–11. doi:10.1176/appi.ajp.158.5.704. PMID 11329390.
[40] Al-Aama, T., Brymer, C., Gutmanis, I., Woolmore-Goodwin, S. M., Esbaugh, J. and Dasgupta, M. , Melatonin decreases delirium in elderly
patients: A randomized, placebo-controlled trial. International Journal of Geriatric Psychiatry, n/a. doi: 10.1002/gps.2582
[41] Tjon Pian Gi CV, Broeren JP, Starreveld JS, Versteegh FG (2003). "Melatonin for treatment of sleeping disorders in children with attention
deficit/hyperactivity disorder: a preliminary open label study". Eur J Pediatr. 162 (7): 554–555. doi:10.1007/s00431-003-1207-x.
PMID 12783318.
[42] Bellipanni G, DI Marzo F, Blasi F, Di Marzo A (2005). "Effects of melatonin in perimenopausal and menopausal women: our personal
experience". Ann N Y Acad Sci 1057 (Dec): 393–402. doi:10.1196/annals.1356.030. PMID 16399909.
[43] "Melatonin" (http:/ / sleepdisorders. about. com/ cs/ melatonin/ a/ melatonin_4. htm). About.com: Sleep Disorders: 4. .
40
Melatonin
[44] Terzolo M, et al., 1993."Evening administration of melatonin enhances the pulsatile secretion of prolactin but not of LH and TSH in
normally cycling women." Clinical Endocrinology, 39(2):185–191.
[45] http:/ / www. wddty. com/ the-infertility-drug. html
[46] Jahnke G, Marr M, Myers C, Wilson R, Travlos G, Price C (1999). "Maternal and developmental toxicity evaluation of melatonin
administered orally to pregnant Sprague-Dawley rats". Toxicological Sciences 50 (2): 271–9. doi:10.1093/toxsci/50.2.271. PMID 10478864.
[47] Dodick D, Capobianco D (2001). "Treatment and management of cluster headache". Curr Pain Headache Rep 5 (1): 83–91.
doi:10.1007/s11916-001-0015-0. PMID 11252143.
[48] Gagnier J (2001). "The therapeutic potential of melatonin in migraines and other headache types". Altern Med Rev 6 (4): 383–9.
PMID 11578254.
[49] http:/ / www. nimh. nih. gov/ press/ sad-melatonin. cfm
[50] Bhattacharjee, Yudhijit (14 September 2007). "Is Internal Timing Key to Mental Health?" (http:/ / www. ohsu. edu/ ohsuedu/ academic/
som/ images/ Al-Lewy-Science. pdf) (PDF). ScienceMag (AAAS) 317: 1488–90. . Retrieved 2008-02-18.
[51] Lewy AJ, Nurnberger JI, Wehr TA (June 1985). "Supersensitivity to light: possible trait marker for manic-depressive illness" (http:/ / ajp.
psychiatryonline. org/ cgi/ pmidlookup?view=long& pmid=4003592). Am J Psychiatry 142 (6): 725–7. PMID 4003592. .
[52] Whalley LJ, Perini T, Shering A, Bennie J (July 1991). "Melatonin response to bright light in recovered, drug-free, bipolar patients".
Psychiatry Res 38 (1): 13–9. doi:10.1016/0165-1781(91)90048-T. PMID 1658841.
[53] Mills E, Wu P, Seely D,4; Guyatt G, E; Wu, P; Seely, D; Guyatt, G (2005). "Melatonin in the treatment of cancer: a systematic review of
randomized controlled trials and meta-analysis" (http:/ / pt. wkhealth. com/ pt/ re/ jpin/ abstract. 00005208-200511000-00005. htm). Journal
of pineal research 39 (4): 360–6. doi:10.1111/j.1600-079X.2005.00258.x. PMID 16207291. .
[54] CCNM. Current Research (http:/ / www. ccnm. edu/ ?q=current_research).
[55] Navara, KJ; Nelson, RJ (2007). "The dark side of light at night: physiological, epidemiological, and ecological consequences" (http:/ / www.
psy. ohio-state. edu/ nelson/ documents/ JPinealRes2007. pdf) (Review, PDF: full text). J. Pineal Res. 43 (43): 215–224.
doi:10.1111/j.1600-079X.2007.00473.x. PMID 17803517. . Retrieved 2008-05-07.
[56] Schernhammer E, Rosner B, Willett W, Laden F, Colditz G, Hankinson S (2004). "Epidemiology of urinary melatonin in women and its
relation to other hormones and night work". Cancer Epidemiol Biomarkers Prev 13 (62): 936–43. PMID 15184249.
[57] Koppisetti S, Jenigiri B, Terron MP (October 2008). "Reactive oxygen species and the hypomotility of the gall bladder as targets for the
treatment of gallstones with melatonin: a review". Dig. Dis. Sci. 53 (10): 2592–603. doi:10.1007/s10620-007-0195-5. PMID 18338264.
[58] Tan D, Manchester LC, Reiter RJ, Qi W, Hanes MA, Farley NJ (October 1999). "High physiological levels of melatonin in the bile of
mammals" (http:/ / linkinghub. elsevier. com/ retrieve/ pii/ S0024320599005196). Life Sci. 65 (23): 2523–9.
doi:10.1016/S0024-3205(99)00519-6. PMID 10622237. .
[59] Weishaupt, J. H.; et al (November 2006). "Reduced oxidative damage in ALS by high-dose enteral melatonin treatment" (http:/ / www. ncbi.
nlm. nih. gov/ pubmed/ 17014688). J Pineal Res. 41 (4): 313–23. doi:10.1111/j.1600-079X.2006.00377.x. PMID 17014688. . Retrieved
2010-07-03.
[60] Wolden-Hanson T, Mitton DR, McCants RL, Yellon SM, Wilkinson CW, Matsumoto AM, Rasmussen Daily melatonin administration to
middle-aged male rats suppresses body weight, intraabdominal adiposity, and plasma leptin and insulin independent of food intake and total
body fat.Endocrinology.141 (2)487-497, 2000 PMID=10650927.
[61] Tan DX, Manchester LC, Fuentes-Broto L, Paredes SD, Reiter RJ (March 2011). "Significance and application of melatonin in the
regulation of brown adipose tissue metabolism: relation to human obesity". Obesity Reviews 12 (3): 167–88.
doi:10.1111/j.1467-789X.2010.00756.x. PMID 20557470.
[62] Vijayalaxmi, Meltz ML, Reiter RJ, Herman TS, Kumar KS (March 1999). "Melatonin and protection from whole-body irradiation: survival
studies in mice". Mutation research 425 (1): 21–7. PMID 10082913.
[63] Vijayalaxmi, Reiter RJ, Herman TS, Meltz ML (December 1996). "Melatonin and radioprotection from genetic damage: in vivo/in vitro
studies with human volunteers". Mutation research 371 (3–4): 221–8. doi:10.1016/S0165-1218(96)90110-X. PMID 9008723.
[64] Vijayalaxmi, Reiter RJ, Herman TS, Meltz ML (February 1998). "Melatonin reduces gamma radiation-induced primary DNA damage in
human blood lymphocytes". Mutation research 397 (2): 203–8. PMID 9541644.
[65] Topkan E, Tufan H, Yavuz AA, et al. (October 2008). "Comparison of the protective effects of melatonin and amifostine on
radiation-induced epiphyseal injury". International Journal of Radiation Biology 84 (10): 796–802. doi:10.1080/09553000802389678.
PMID 18979313.
[66] Tan DX, Manchester LC, Terron MP, Flores LJ, Reiter RJ (January 2007). "One molecule, many derivatives: A never-ending interaction of
melatonin with reactive oxygen and nitrogen species?". Journal of Pineal Research 42 (1): 28–42. doi:10.1111/j.1600-079X.2006.00407.x.
PMID 17198536.
[67] Shirazi A, Ghobadi G, Ghazi-Khansari M (July 2007). "A radiobiological review on melatonin: a novel radioprotector". J. Radiat. Res. 48
(4): 263–72. doi:10.1269/jrr.06070. PMID 17641465.
[68] From Ross' Histology and Wheater's Functional Histology.
[69] Sotthibundhu A, Phansuwan-Pujito P, Govitrapong P (October 2010). "Melatonin increases proliferation of cultured neural stem cells
obtained from adult mouse subventricular zone". Journal of Pineal Research 49 (3): 291–300. doi:10.1111/j.1600-079X.2010.00794.x.
PMID 20663047.
[70] Kunz D, Bes F (March 2001). "Exogenous melatonin in periodic limb movement disorder: an open clinical trial and a hypothesis". Sleep 24
(2): 183–7. PMID 11247054.
41
Melatonin
[71] Basu P.P., Pacana T., Shah N., Hampole H., Krishnaswamy N., Rayapudi K. "Role of melatonin in colonic motility in irritable bowel
syndrome - Constipation MIMI-C-a double blinded randomized placebocontrol clinical trial" Neurogastroenterology and Motility 2010 22
SUPPL. 1 (67)
[72] Buscemi, Nina; Vandermeer, B; Hooton, N; Pandya, R; Tjosvold, L; Hartling, L; Vohra, S; Klassen, TP et al. (2006-02-18). "Efficacy and
safety of exogenous melatonin for secondary sleep disorders and sleep disorders accompanying sleep restriction: meta-analysis" (http:/ / www.
bmj. com/ cgi/ content/ full/ 332/ 7538/ 385). BMJ 332 (7538): 385–393. doi:10.1136/bmj.38731.532766.F6. PMC 1370968.
PMID 16473858. . Retrieved 2008-05-17.
[73] Buscemi N, Vandermeer B, Hooton N, et al. (December 2005). "The efficacy and safety of exogenous melatonin for primary sleep
disorders. A meta-analysis". Journal of General Internal Medicine 20 (12): 1151–8. doi:10.1111/j.1525-1497.2005.0243.x. PMC 1490287.
PMID 16423108.
[74] "melatonin cautions" (http:/ / www. melatonin. com/ melatonin-cautions. php). www.melatonin.com. .
[75] Zhdanova, Irinia V., Richard J. Wurtman, Meredith M. Regan, et al. "Melatonin Treatment for Age-Related Insomnia." Journal of Clinical
Endocrinology & Metabolism 86.10 (2001): 4727-4730.
[76] Sack, Robert L.; Brandes, RW; Kendall, AR; Lewy, AJ (12 October 2000). "Entrainment of Free-Running Circadian Rhythms by Melatonin
in Blind People" (http:/ / content. nejm. org/ cgi/ content/ abstract/ 343/ 15/ 1070). The NEW ENGLAND JOURNAL of MEDICINE 343 (15):
1070–1077. doi:10.1056/NEJM200010123431503. PMID 11027741. . Retrieved 2008-04-13.
[77] Morera A, Henry M, de La Varga M (2001). "Safety in melatonin use". Actas Esp Psiquiatr 29 (5): 334–7. PMID 11602091.
[78] Terry PD, Villinger F, Bubenik GA, Sitaraman SV (January 2009). "Melatonin and ulcerative colitis: evidence, biological mechanisms, and
future research". Inflammatory Bowel Diseases 15 (1): 134–40. doi:10.1002/ibd.20527. PMID 18626968.
[79] Penn State College of Medicine, Milton S. Hershey Medical Center (September 2003). "Study Shows Melatonin Supplements May Make
Standing A Hazard For The Cardiovascular-Challenged" (http:/ / www. hmc. psu. edu/ news/ pr/ 2003/ sept/ Ray_melatonin. doc) (DOC).
Press release. . Retrieved 2006-07-21. (MS Word Format)
[80] "Melatonin Information from Drugs.com" (http:/ / www. drugs. com/ melatonin. html). .
[81] Burkhardt S, Tan DX, Manchester LC, Hardeland R, Reiter RJ (October 2001). "Detection and quantification of the antioxidant melatonin in
Montmorency and Balaton tart cherries (Prunus cerasus).". Journal of agricultural and food chemistry 49 (10): 4898–902.
doi:10.1021/jf010321+. PMID 11600041.
[82] Tan DX, Hardeland R, Manchester LC, et al. (August 2010). "The changing biological roles of melatonin during evolution: from an
antioxidant to signals of darkness, sexual selection and fitness". Biological Reviews 85 (3): 607–23. doi:10.1111/j.1469-185X.2009.00118.x.
PMID 20039865.
[83] Lincoln GA, Andersson H, Loudon A (October 2003). "Clock genes in calendar cells as the basis of annual timekeeping in mammals—a
unifying hypothesis". The Journal of Endocrinology 179 (1): 1–13. doi:10.1677/joe.0.1790001. PMID 14529560.
[84] Arendt J, Skene DJ (2005). "Melatonin as a chronobiotic". Sleep Med Rev 9 (1): 25–39. doi:10.1016/j.smrv.2004.05.002. PMID 15649736.
"Exogenous melatonin has acute sleepiness-inducing and temperature-lowering effects during 'biological daytime', and when suitably timed (it
is most effective around dusk and dawn) it will shift the phase of the human circadian clock (sleep, endogenous melatonin, core body
temperature, cortisol) to earlier (advance phase shift) or later (delay phase shift) times.".
[85] CM Chaturvedi (1984). "Effect of Melatonin on the Adrenl and Gonad of the Common Mynah Acridtheres tristis" (http:/ / www. publish.
csiro. au/ paper/ ZO9840803. htm). Australian Journal of Zoology 32 (6): 803–809. doi:10.1071/ZO9840803. .
[86] Chen H (1981). "Spontaneous and melatonin-induced testicular regression in male golden hamsters: augmented sensitivity of the old male to
melatonin inhibition". Neuroendocrinology 33 (1): 43–6. doi:10.1159/000123198. PMID 7254478.
[87] Nayak SK, Jegla T, Panda S (January 2007). "Role of a novel photopigment, melanopsin, in behavioral adaptation to light". Cell. Mol. Life
Sci. 64 (2): 144–54. doi:10.1007/s00018-006-5581-1. PMID 17160354.
[88] Roberts JE (2005). "Update on the positive effects of light in humans". Photochem. Photobiol. 81 (3): 490–2.
doi:10.1562/2004-12-02-IR-391. PMID 15656701.
[89] Richardson G (2005). "The human circadian system in normal and disordered sleep". J Clin Psychiatry. 66 Suppl 9: 3–9; quiz 42–3.
PMID 16336035.
[90] Perreau-Lenz S, Pévet P, Buijs R, Kalsbeek A (2004). "The biological clock: the bodyguard of temporal homeostasis". Chronobiol Int 21
(1): 1–25. doi:10.1081/CBI-120027984. PMID 15129821.
[91] Ardura, Julio; Gutierrez, R; Andres, J; Agapito, T (2002). "Emergence and Evolution of the Circadian Rhythm of Melatonin in Children"
(http:/ / content. karger. com/ ProdukteDB/ produkte. asp?Doi=68571) (Free abstract). Hormone Research (S. Karger AG, Basel) 59 (2):
66–72. doi:10.1159/000068571. PMID 12589109. . Retrieved 2009-11-08.
[92] "www.ncbi.nlm.nih.gov/pubmed/3783419" (http:/ / www. ncbi. nlm. nih. gov/ pubmed/ 3783419). . Retrieved February 9, 2011. Human
melatonin production decreases with age, 1986, Sack RL, Lewy AJ, Erb DL, Vollmer WM, Singer CM.
[93] "kidshealth.org/teen/your_body/take_care/how_much_sleep.html" (http:/ / kidshealth. org/ teen/ your_body/ take_care/ how_much_sleep.
html). . Retrieved April 26, 2011. Why Aren't Teens Getting Enough Sleep? 2009, Mary L. Gavin, Mena T. Scavina.
[94] Terman
[95] Wirz-Justice, A; Benedetti, F; Terman, M (2009). Chronotherapeutics for Affective Disorders: A Clinician’s Manual for Light and Wake
Therapy. Basel: Karger. ISBN 978-3-8055-9120-1.
[96] Brainard GC, Hanifin JP, Greeson JM, Byrne B, Glickman G, Gerner E, Rollag (August 15, 2001). "Action spectrum for melatonin
regulation in humans: evidence for a novel circadian photoreceptor". J Neurosci. 15;21 (16): 6405–12. PMID 11487664.
42
Melatonin
[97] Kayumov L, Casper RF, Hawa RJ, Perelman B Chung SA, Sokalsky S, Shipiro (May 2005). "Blocking low-wavelength light prevents
nocturnal melatonin suppression with no adverse effect on performance during simulated shift work". J Clin Endocrinol Metab. 90 (5):
2755–61. doi:10.1210/jc.2004-2062. PMID 15713707.
[98] Tan D.X., L.D. Chen, B. Poeggeler, L.C. Manchester, R.J. Reiter (1993) Melatonin: a potent, endogenous hydroxyl radical scavenger.
Endocrine J. 1: 57-60
[99] Dun-Xian Tan, Lucien C. Manchester, Maria P. Terron, Luis J. Flores, Russel J. Reiter (2007). "One molecule, many derivatives: a
never-ending interaction of melatonin with reactive oxygen and nitrogen species?". Journal of Pineal Research 42 (1): 28–42.
doi:10.1111/j.1600-079X.2006.00407.x. PMID 17198536.
[100] Poeggeler B, Saarela S, Reiter RJ (1994). "Melatonin—a highly potent endogenous radical scavenger and electron donor: new aspects of
the oxidation chemistry of this indole accessed in vitro". Ann. N. Y. Acad. Sci. 738 (1): 419–20. doi:10.1111/j.1749-6632.1994.tb21831.x.
PMID 7832450.
[101] Tan DX, Manchester LC, Reiter RJ, Qi W, Karbownik M, Calvo JR (2000). "Significance of melatonin in anti oxidative defense system:
reactions and products". Biol Signals Recept 9 (3–4): 137–59. doi:10.1159/000014635. PMID 10899700.
[102] Karbownik M, Reiter R, Cabrera J, Garcia J (2001). "Comparison of the protective effect of melatonin with other antioxidants in the
hamster kidney model of estradiol-induced DNA damage". Mutat Res 474 (1–2): 87–92. doi:10.1016/S0027-5107(00)00164-0.
PMID 11239965.
[103] Tütüncüler F, Eskiocak S, Başaran UN, Ekuklu G, Ayvaz S, Vatansever U (2005). "The protective role of melatonin in experimental
hypoxic brain damage". Pediatr Int 47 (4): 434–9. doi:10.1111/j.1442-200x.2005.02085.x. PMID 16091083.
[104] Ward Dean, John Morgenthaler, Steven William Fowkes (1993). Smart Drugs II: The Next Generation : New Drugs and Nutrients to
Improve Your Memory and Increase Your Intelligence (Smart Drug Series, V. 2). Smart Publications. ISBN 0-9627418-7-6.
[105] Anisimov V, Alimova I, Baturin D, Popovich I, Zabezhinski M, Rosenfeld S, Manton K, Semenchenko A, Yashin A (2003).
"Dose-dependent effect of melatonin on life span and spontaneous tumor incidence in female SHR mice". Exp Gerontol 38 (4): 449–61.
doi:10.1016/S0531-5565(02)00240-1. PMID 12670632.
[106] Oaknin-Bendahan S, Anis Y, Nir I, Zisapel N (1995). "Effects of long-term administration of melatonin and a putative antagonist on the
ageing rat". Neuroreport 6 (5): 785–8. doi:10.1097/00001756-199503270-00020. PMID 7605949.
[107] Carrillo-Vico A, Guerrero J, Lardone P, Reiter R (2005). "A review of the multiple actions of melatonin on the immune system".
Endocrine 27 (2): 189–200. doi:10.1385/ENDO:27:2:189. PMID 16217132.
[108] Arushanian E, Beier E (2002). "Immunotropic properties of pineal melatonin". Eksp Klin Farmakol 65 (5): 73–80. PMID 12596522.
[109] Carrillo-Vico A, Reiter RJ, Lardone PJ (2006). "The modulatory role of melatonin on immune responsiveness". Curr Opin Investig Drugs
7 (5): 423–31. PMID 16729718.
[110] Maestroni GJ (2001). "The immunotherapeutic potential of melatonin". Expert Opin Investig Drugs 10 (3): 467–76.
doi:10.1517/13543784.10.3.467. PMID 11227046.
[111] Maestroni G (1999). "Therapeutic potential of melatonin in immunodeficiency states, viral diseases, and cancer". Adv Exp Med Biol 467:
217–26. PMID 10721059.
[112] Carrillo-Vico A, Lardone PJ, Fernández-Santos JM (2005). "Human lymphocyte-synthesized melatonin is involved in the regulation of the
interleukin-2/interleukin-2 receptor system". J. Clin. Endocrinol. Metab. 90 (2): 992–1000. doi:10.1210/jc.2004-1429. PMID 15562014.
[113] Cutolo M, Maestroni GJ (2005). "The melatonin-cytokine connection in rheumatoid arthritis". Ann. Rheum. Dis. 64 (8): 1109–11.
doi:10.1136/ard.2005.038588. PMC 1755599. PMID 16014678.
[114] Arias J, Melean E, Valero N, et al. (March 2003). "[Effect of melatonin on lymphocyte proliferation and production of interleukin-2 (IL-2)
and interleukin-1 beta (IL-1 beta) in mice splenocytes]" (in Spanish; Castilian). Invest Clin 44 (1): 41–50. PMID 12703182.
[115] Lewis, Alan (1999). Melatonin and the Biological Clock. McGraw-Hill. pp. 23. ISBN 0879837349.
[116] Melke J, Botros HG, Chaste P (2008). "Abnormal melatonin synthesis in autism spectrum disorders". Mol Psychiatry 13 (1): 90–8.
doi:10.1038/sj.mp.4002016. PMC 2199264. PMID 17505466.
[117] Wright, B; Sims, D, Smart, S, Alwazeer, A, Alderson-Day, B, Allgar, V, Whitton, C, Tomlinson, H, Bennett, S, Jardine, J, McCaffrey, N,
Leyland, C, Jakeman, C, Miles, J (2011 Feb). "Melatonin versus placebo in children with autism spectrum conditions and severe sleep
problems not amenable to behaviour management strategies: a randomised controlled crossover trial". Journal of autism and developmental
disorders 41 (2): 175–84. doi:10.1007/s10803-010-1036-5. PMID 20535539.
[118] University of Maryland Medical Center. “Melatonin.” 2011. http:/ / www. umm. edu/ altmed/ articles/ melatonin-000315. htm
[119] Bondy, S.C., K.G. Sharman, Y-W. Yuan-Wen Ge, et al. "Age-related changes in murine CNS mRNA gene expression are modulated by
dietary melatonin." American Aging Association. Irvine, CA, University of California. 2003.
[120] University of Granada. "High Melatonin Content Can Help Delay Aging, Mouse Study Suggests." ScienceDaily 24 April 2007. 13
February 2011 http:// www.sciencedaily.com /releases/2007/04/070424062819.htm
[121] Turek FW, Gillette MU (November 2004). "Melatonin, sleep, and circadian rhythms: rationale for development of specific melatonin
agonists". Sleep Med. 5 (6): 523–32. doi:10.1016/j.sleep.2004.07.009. PMID 15511698.
[122] Wade AG, Ford I, Crawford G (October 2007). "Efficacy of prolonged release melatonin in insomnia patients aged 55–80 years: quality of
sleep and next-day alertness outcomes". Curr Med Res Opin 23 (10): 2597–605. doi:10.1185/030079907X233098. PMID 17875243.
[123] Zhdanova I, Wurtman R, Regan M, Taylor J, Shi J, Leclair O (2001). "Melatonin treatment for age-related insomnia". J Clin Endocrinol
Metab 86 (10): 4727–30. doi:10.1210/jc.86.10.4727. PMID 11600532.
43
Melatonin
[124] Lewy AJ, Emens JS, Sack RL, Hasler BP, Bernert RA (2002). "Low, but not high, doses of melatonin entrained a free-running blind person
with a long circadian period". Chronobiol Int. 19 (3): 649–58. doi:10.1081/CBI-120004546. PMID 12069043.
[125] Sack, Robert L.; Richard W. Brandes, Adam R. Kendall, Alfred J. Lewy (October 2000). "Entrainment of Free-Running Circadian
Rhythms by Melatonin in Blind People" (http:/ / www. nejm. org/ doi/ full/ 10. 1056/ NEJM200010123431503#t=article) (Full text). New
England Journal of Medicine 343 (343): 1070–77. doi:10.1056/NEJM200010123431503. . Retrieved 2010-12-01.
[126] Filadelfi AM, Castrucci AM (May 1996). "Comparative aspects of the pineal/melatonin system of poikilothermic vertebrates". Journal of
Pineal Research 20 (4): 175–86. doi:10.1111/j.1600-079X.1996.tb00256.x. PMID 8836950.
[127] Sugden D, Davidson K, Hough KA, Teh MT (October 2004). "Melatonin, melatonin receptors and melanophores: a moving story".
Pigment Cell Research 17 (5): 454–60. doi:10.1111/j.1600-0749.2004.00185.x. PMID 15357831.
[128] Lerner AB, Case JD, Takahashi Y (1960). "Isolation of melatonin and 5-methoxyindole-3-acetic acid from bovine pineal glands". J Biol
Chem 235: 1992–7. PMID 14415935.
[129] Lynch HJ, Wurtman RJ, Moskowitz MA, Archer MC, Ho MH (January 1975). "Daily rhythm in human urinary melatonin". Science 187
(4172): 169–71. doi:10.1126/science.1167425. PMID 1167425.
[130] Arendt, Josephine (August 2005). "Melatonin: Characteristics, Concerns, and Prospects" (Review). J Biol Rhythms (SagePub) 20 (4):
291–303. doi:10.1177/0748730405277492. PMID 16077149. "There is very little evidence in the short term for toxicity or undesirable effects
in humans. The extraordinary “hype” of the miraculous powers of melatonin in the recent past did a disservice to acceptance of its genuine
benefits.".
[131] Arendt, Josephine (12). "Melatonin, Circadian Rhythms, and Sleep" (Editorial). N Engl J Med (The New NEJM) 343 (15): 1114–1116.
doi:10.1056/NEJM200010123431510. PMID 11027748.
[132] U.S. Food and Drug Administration (2007-06-22). "FDA Issues Dietary Supplements Final Rule" (http:/ / www. fda. gov/ NewsEvents/
Newsroom/ PressAnnouncements/ 2007/ ucm108938. htm). Press release. . Retrieved 2009-08-04.
[133] Hoebert M, van der Heijden KB, van Geijlswijk IM, Smits MG (August 2009). "Long-term follow-up of melatonin treatment in children
with ADHD and chronic sleep onset insomnia". Journal of Pineal Research 47 (1): 1–7. doi:10.1111/j.1600-079X.2009.00681.x.
PMID 19486273.
[134] Medical News Today (http:/ / www. medicalnewstoday. com/ articles/ 69195. php) Circadin (Prolonged-Release Melatonin) For Primary
Insomnia Recommended For Approval In The EU (27 Apr 2007)
External links
• Melatonin information (http://www.nlm.nih.gov/medlineplus/druginfo/natural/patient-melatonin.html) from
MedlinePlus
• Melatonin entry in TiHKAL • info (http://tihkal.info/read.php?domain=tk&id=35)
• What Is Melatonin - Sleep Aid and Mental Health Remedy (http://www.melatoninsideeffects.org/
what-is-melatonin/)
44
Mephedrone
45
Mephedrone
Mephedrone
Systematic (IUPAC) name
(RS)-2-methylamino-1-(4-methylphenyl)propan-1-one
[1] :5
Identifiers
[2]
CAS number
1189805-46-6
[1]
1189726-22-4 (HCl) :5
ATC code
None
PubChem
CID 29982893
ChemSpider
21485694
Synonyms
4-methyl-N-methylcathinone; 2-methylamino-1-p-tolylpropan-1-one
[3]
[4]
[5]
Chemical data
Formula
C H NO
Mol. mass
177.242 g/mol
SMILES
eMolecules
11
15
[6]
& PubChem
[7]
Therapeutic considerations
Pregnancy cat. ?
Legal status
See legal status section
Mephedrone
46
Routes
Oral, Insufflation, IV, rectal,
[8]
[9]
smoking
(what is this?) (verify)
[10]
Mephedrone, also known as 4-methylmethcathinone (4-MMC), or 4-methylephedrone, is a synthetic stimulant
and entactogen drug of the amphetamine and cathinone classes. Slang names include M4, Smeech, meph,[11]
drone,[12] and MCAT.[13] It is reportedly manufactured in China and is chemically similar to the cathinone
compounds found in the khat plant of eastern Africa. It comes in the form of tablets or a powder, which users can
swallow, snort or inject, producing similar effects to MDMA, amphetamines, and cocaine.
As well as producing the intended stimulant effects, negative side effects occur when mephedrone is used, with teeth
grinding the most common. The metabolism of mephedrone has been studied in rats and humans, and the metabolites
can be detected in urine after usage. Nothing is known about the potential neurotoxicity of mephedrone, but
scientists have suggested possible dangers associated with its use based on its similarity to other drugs. Several
people have died after consuming mephedrone, but some deaths that the media attributed to the drug were later
determined to have been caused by other factors.
Mephedrone was first synthesised in 1929 but did not become widely known until it was rediscovered in 2003. By
2007 mephedrone was reported to be available for sale on the internet, by 2008 law enforcement agencies had
become aware of the compound and by 2010 it had been reported in most of Europe, becoming particularly prevalent
in the United Kingdom. Mephedrone was first made illegal in Israel in 2008, followed by Sweden later that year. In
2010 it was made illegal in many European countries and in December 2010, the EU ruled it illegal across Europe.
In Australia, New Zealand, and the USA it is considered an analog of other illegal drugs and can be controlled by
laws similar to the Federal Analog Act. In the USA, this only applies if the drug is sold for human consumption,
allowing it to be sold legally if labelled as 'plant food' or 'bath salts'.
History
Mephedrone is one of hundreds of designer drugs or legal highs that have been reported in recent years, alongside
with herbs such as Salvia divinorum, artificial chemicals such as synthetic cannabis and semi-synthetic substances
such as methylhexaneamine. These drugs are primarily developed to avoid being controlled by laws against illegal
drugs, thus giving them the label of designer drugs.[14] According to the European Monitoring Centre for Drugs and
Drug Addiction, the synthesis of mephedrone was first reported in 1929 by Saem de Burnaga Sanchez in the Bulletin
de la Société Chimique de France, under the name "toluyl-alpha-monomethylaminoethylcetone",[1] :17[15] but the
compound remained an obscure product of academia until 2003, when it was "re-discovered" and publicised by an
underground chemist on The Hive website, working under the pseudonym "Kinetic."[16] Kinetic posted on the site,
"I’ve been bored over the last couple of days and had a few fun reagents lying around, so I thought I’d try and make
some 1-(4-methylphenyl)-2-methylaminopropanone hydrochloride, or 4-methylmethcathinone." before going on to
describe that after taking it they had a "fantastic sense of well-being that I haven’t got from any drug before except
my beloved Ecstasy."[17] A drug similar to mephedrone, containing cathinone, was sold legally in Israel from around
2004, under the name hagigat. When this was made illegal, the cathinone was modified and the new products were
sold by the Israeli company, Neorganics.[18] [19] [20] The products had names such as Neodoves pills, but the range
was discontinued in January 2008 after the Israeli government made mephedrone illegal.[12] [21] [22] The Psychonaut
Research Project, an EU organisation that searches the internet for information regarding new drugs, first identified
mephedrone in 2008. Their research suggests that the drug first became available to purchase on the internet in 2007,
when it was also discussed on internet forums.[14] [23] Mephedrone was first seized in France in May 2007 after
police sent a tablet that they assumed to be ecstasy to be analysed, with the discovery published in a paper titled "Is
4-methylephedrone, an “Ecstasy” of the twenty first century?"[24] Mephedrone was reported as having been sold as
ecstasy in the Australian city of Cairns, along with ethylcathinone in 2008.[25] [26] An annual survey of regular
ecstasy users in Australia in 2010 found that 21% of those surveyed had used mephedrone, with 17% having done so
Mephedrone
in the previous six months. The price they paid per gram varied from A$16 to $320.[9] Europol noted that they
became aware of it in 2008, after it was found in Denmark, Finland and the UK.[27] The Drug Enforcement Agency
noted it was present in the United States in July 2009.[28] By May 2010, mephedrone had been detected in every one
of the 22 EU member states that reported to Europol, as well as in Croatia and Norway.[1] :21 The Daily Telegraph
reported in April 2009 that it was manufactured in China, but it has since been made illegal there.[29] [30] In March
2009, Druglink magazine reported that it only cost a "couple hundred pounds" to synthesise a kilogram of
mephedrone,[18] the same month, The Daily Telegraph reported that manufacturers were making "huge amounts of
money" from selling it.[31] In January 2010 Druglink magazine reported that dealers in Britain spent £2,500 to ship
one kilogram from China but could sell it for £10 a gram making a profit of £7,500.[17] [32] A later report, in March
2010, stated that the wholesale price of mephedrone was £4000 per kilogram.[33]
In the United Kingdom
Between the summer of 2009 and March 2010 the use of mephedrone
grew rapidly in the UK, with it becoming readily available at music
festivals, head shops and on the internet.[35] A survey of Mixmag
readers in 2009, found that it was the fourth most popular street drug in
the United Kingdom, behind cannabis, cocaine, and ecstasy.[33] The
drug is used by a diverse range of social groups. Whilst the evidence is
anecdotal, researchers, charity workers, teachers and users have
reported widespread and increasing use of the drug. The drug's rapid
The number of samples analysed by the Forensic
growth in popularity was believed to be related to both its availability
Science Service of seized MDMA, piperazines
[35]
and legality.
Fiona Measham, a criminologist at The University of
and cathinones between the third quarter of 2005
Lancaster, believes that the emergence of mephedrone was also related
and the first quarter of 2010. MDMA seizures in
blue, piperazine seizures in orange and cathinone
to the decreasing purity of ecstasy and cocaine on sale in the UK,[35] a
[34]
seizures in purple
view reinforced in a report by the National Treatment Agency for
Substance Misuse.[36] The average cocaine purity fell from 60% in
1999 to 22% in 2009 and about half of ecstasy pills seized in 2009 contained no MDMA,[37] and by June 2010,
almost all pills seized in the UK, contained no MDMA.[38] A similar pattern was observed in the Netherlands, with
the number of ecstasy tablets containing no MDMA rising from 10% in mid 2008 to 60% by mid 2009 and
mephedrone being detected in 20% of ecstasy tablets by mid 2009.[39] The decrease of MDMA was thought in part,
to be due to the seizure of 33 tonnes of sassafras oil, the precursor to MDMA, in Cambodia in June 2008, which
could have been used to make 245 million doses of MDMA.[17] According to John Ramsey, a toxicologist at St
George's, University of London, the emergence of mephedrone was also related to the UK government banning the
benzylpiperazine class of drugs in December 2009.[18] [40] gamma-Butyrolactone (GBL), another previously "legal
high" was also banned in August 2009, despite concerns that it would be replaced by other drugs.[41] By December
2009, mephedrone was available on at least 31 websites based in the UK and by March 2010 there were at least 78
online shops; half of which sold amounts of less than 200 grams and half that also sold bulk quantities. The price per
gram varied from £9.50 to £14.[1] :11 Between July 2009 and February 2010, UK health professionals accessed the
National Poisons Information Service's (NPIS) entry on mephedrone 1664 times and made 157 telephone inquiries;
the requests increased month on month over this period. In comparison over a similar time period, the entries for
cocaine and MDMA were accessed approximately 2400 times.[42] After mephedrone was made illegal, the number
of inquiries to the NPIS fell substantially, to only 19 in June 2010.[43]
Media organisations including the BBC and The Guardian, as well as a news section in the Annals of Botany[44]
(later corrected[45] ), incorrectly reported that mephedrone was commonly used as a plant fertiliser. In fact sellers of
the drug described it as "plant food" because it was illegal to sell the compound for human consumption.[37] In late
2009, UK newspapers began referring to the drug as meow or miaow (sometimes doubled as meow meow or miaow
47
Mephedrone
miaow), a name that was almost unknown on the street at the time.[46] In November 2009, the tabloid newspaper,
The Sun published a story stating that a man had ripped off his own scrotum whilst using mephedrone,[47] but this
story was later shown to be an online hoax.[48] Other myths that the media often repeated during 2010 were that
mephedrone had led to the deaths of over 20 people, that teachers were unable to confiscate the drug off pupils and
that the government was too slow to ban the drug.[49] Parallels were drawn between the media coverage of
mephedrone and a piece of satire by Chris Morris in 1997 on Brass Eye, when he tricked public figures into talking
of the dangers of taking the fictional legal drug "cake".[50] The Advisory Council on the Misuse of Drugs (ACMD)
have suggested that the media coverage of the drug led to increased usage of it.[51] Jon Silverman, a former BBC
Home Affairs Correspondent, has written two articles discussing how the media had a strong influence over the
governments drugs policy, particularly in that the government wished to demonstrate that they were being “tough” on
drugs.[41] [52]
A survey of 1000 secondary school pupils and university students in Tayside, conducted in February 2010, found
that 20% of them had previously taken mephedrone. Although at the time it was available legally over the internet,
only 10% of users reported purchasing it online, with most purchasing it from street dealers. Of those who had used
mephedrone, 97% said that it was easy or very easy to obtain. Around 50% of users reported at least one negative
effect associated with the use of mephedrone, of which teeth grinding is the most common.[53] Detailed interviews
with users in Northern Ireland similarly, found that few purchased mephedrone online, with most interviewees citing
concerns that their address would be traced or that family members could intercept the package.[14]
On 30 March 2010, Alan Johnson, the then Home Secretary, announced that mephedrone would be made illegal
"within weeks" after the ACMD sent him a report on the use of cathinones.[54] [55] The legislation would make all
cathinones illegal which Johnson said would "stop unscrupulous manufacturers and others peddling different but
similarly harmful drugs".[56] The ACMD had run into problems with the UK Government in 2009 regarding drugs
policy, after the government did not follow the advice of the ACMD to reclassify ecstasy and cannabis, culminating
in the dismissal of the ACMD chairman, David Nutt after he reiterated the ACMD's findings in an academic
lecture.[57] Several members resigned after he was sacked and prior to the announcement that mephedrone was to be
banned the trend continued when Dr Polly Taylor resigned, saying she "did not have trust" in the way the
government would use the advice given by the ACMD.[58] Eric Carlin, a member of the ACMD and former
chairman of the English Drug Education Forum, also resigned after the announcement. He said that the decision by
the Home Secretary was "unduly based on media and political pressure" and there was "little or no discussion about
how our recommendation to classify this drug would be likely to impact on young people's behaviour."[59] Some
ex-members of the ACMD, and various charity groups expressed concern over the banning of the drug, arguing it
would inevitably criminalise users, particularly young people.[60] Others expressed concern that the drug would be
left in the hands of black market dealers, who will only compound the problem.[61] Carlin's resignation was
specifically linked to the criminalisation of mephedrone, he stated: "We need to review our entire approach to drugs,
dumping the idea that legally-sanctioned punishments for drug users should constitute a main part of the armoury in
helping to solve our country’s drug problems. We need to stop harming people who need help and support".[62] The
parliamentary debate was held on 8 April, one day after the 2010 general election had been announced, meaning it
was during the so-called "wash-up period" when legislation is passed with little scrutiny. Only one hour was spent
debating the ban and all three parties agreed, meaning that no vote was required.[63] In an interview conducted in
July 2010, when he was no longer a minister, Johnson admitted that the decision to ban mephedrone was sped up
after widespread reporting of deaths caused by the drug and that because the government wished to pass the law,
before parliament was dissolved prior to the upcoming general election.[41] In January 2011 however, Johnson told
the Scunthorpe Telegraph that the decision was based only on information from the ACMD.[64] An editorial in the
April 2010 edition of The Lancet questioned the decision to ban mephedrone, saying that the ACMD did not have
enough evidence to judge the potential harms caused by mephedrone and arguing that policy makers should have
sought to understand why young people took it and how they could be influenced to not take it.[51] Evan Harris, then
the Liberal Democrat science spokesman, stated that the ACMD "was not 'legally constituted'" as required by the
48
Mephedrone
Misuse of Drugs Act, when the report on cathinones was published, since after Taylor resigned it lacked a veterinary
surgeon.[56] In Chemistry World, John Mann professor of chemistry at Queen's University Belfast, suggested that the
UK create a law similar to the Federal Analog Act of the United States, which would have made mephedrone illegal
as an analog of cathinone.[65] In August 2010, James Brokenshire, the Home Office drugs minister, announced plans
to create a new category in the Misuse of Drugs Act, through the Police Reform and Social Responsibility Bill, that
would allow new legal highs to be made temporarily illegal, without the need for a vote in parliament or advice from
the ACMD, as was required to categorise mephedrone.[66] [67] [68]
According to the Independent Scientific Committee on Drugs, since mephedrone was made illegal a street trade in
the drug has emerged, with prices around double those prior to the ban, at £20-£25 per gram.[69] In September 2010,
Druglink reported that the ban had had a mixed effect on mephedrone use, with it decreasing in some areas,
remaining similar in others and becoming more prevalent in some areas.[70] An online survey of 150 users after the
ban, 63% said that they were continuing to use mephedrone, half of those used the same amount and half said they
used less. Compared to previous surveys, more users purchased it off dealers, rather than the internet. The average
price per gram was £16, compared to around £10 before the ban.[71] The 2010 Mixmag survey of 2500 nightclubbers
found that one quarter had used mephedrone in the previous month, that the price had roughly doubled since it was
made illegal and that it was more likely to be cut with other substances.[72] Of those who had already used
mephedrone prior to the ban, 75% had continued to use it after the ban. Of the various drugs used by the survey
participants, it was the most likely for users to have concerns about.[73] Interviews with users in Northern Ireland
also found that the price had roughly doubled since it was made illegal, to around £30 a gram. Rather than the price
rising due to increased scarcity of the drug, it is thought to have risen for two other reasons. Firstly dealers know that
there is still demand for mephedrone, but are aware that supplies of mephedrone may be exhausted in the future.
Secondly, the dealers perceive that customers are likely to be willing to pay more, for an illegal substance.[14]
Professor Shiela Bird, a statistician at the Medical Research Council, has suggested that the ban of mephedrone may
lead to more cocaine related deaths. In the first six months of 2009, the number of cocaine related deaths fell for the
first time in four years, and fewer soldiers tested positive for cocaine in 2009 than in 2008. She suggested that this
may have been due to users switching to mephedrone from cocaine, but cautioned that before full figures are
available for 2009 and 2010, it will be difficult to determine whether mephedrone saved lives, rather than cost
them.[74] [75] Other supposedly legal drugs have filled the gap in the market since mephedrone was made illegal,
including naphyrone (NRG-1) (since made illegal)[76] and Ivory Wave, which has been found to contain MDPV, a
compound made illegal at the same time as mephedrone. However it is possible that some products branded as Ivory
Wave do not contain MDPV.[77] When tested, some products sold six weeks after mephedrone was banned,
advertised as NRG-1, NRG-2 and MDAI were found to be mephedrone.[78]
Effects
There have been no formal published studies into the effects of mephedrone psychological and behavioural effects of
mephedrone on humans nor on animals from which the potential effects could be extrapolated. As a result, the only
information available comes from users themselves and clinical reports of acute mephedrone toxicity.[1] :12
Psychologists at Liverpool John Moores University were to conduct research into the effects of mephedrone on up to
50 students already using the drug, when it was still legal in the UK.[79] At the time the study was proposed, Les
Iversen, the chair of the Advisory Council on the Misuse of Drugs called the experiments "pretty unethical".[80] The
study was discontinued in August 2010, following the change in the legal status of the drug.[81]
49
Mephedrone
Intended effects
Users have reported that mephedrone causes euphoria, stimulation, an enhanced appreciation for music, an elevated
mood, decreased hostility, improved mental function and mild sexual stimulation; these effects are similar to the
effects of cocaine, amphetamines and MDMA. These effects last different amounts of time, depending on the way
the drug is taken. When taken orally, users report they can feel the effects within 15–45 minutes, when snorted the
effects are felt within minutes and peak within half an hour. The effects last for between two and three hours when
taken orally or nasally, but only half an hour if taken intravenously.[1] :12 Out of 70 Dutch users of mephedrone, 58
described it as an overall pleasant experience and 12 described it as an unpleasant experience.[39] A survey of UK
users, who had previously taken cocaine, found that most users found it produced a better quality and longer lasting
high, was less addictive and carried the same risk as using cocaine.[8]
Side effects
The ECMDDA reported that mephedrone can cause various unintended side effects including: poor concentration,
teeth grinding, problems focusing visually, poor short-term memory, hallucinations, delusions, erratic behaviour[1]
:13
and dilated pupils.[82] They noted that the most severe effects appear anecdotally to be linked with high doses or
prolonged usage and that the effects may be due to users taking other intoxicants at the same time. Other effects that
users in internet forums have noted include changes in body temperature, increased heart rate, breathing difficulties,
loss of appetite, increased sweating, discolouration of extremeties, anxiety, paranoia and depression.[1] :13 When
snorted it can also cause nose bleeds, and nose burns.[1] :13[83] A survey conducted by the National Addiction Centre,
UK found that 67% of mephedrone users experienced sweating, 51% suffered from headaches, 43% from heart
palpitations, 27% from nausea and 15% from cold or blue fingers,[84] indicative of vasoconstriction occurring.[42]
Doctors at Guy's Hospital, London reported that of 15 patients they treated after taking mephedrone in 2009, 53%
were agitated, 40% had increased heart rates, 20% had systolic hypertension and 20% had seizures; three required
treatment with benzodiazepines, predominantly to control their agitation. They reported that none of their patients
suffered from cold or blue peripheries, contrary to other reports. Nine out of the 15 of patients had a Glasgow Coma
Scale (GCS) of 15 indicating that they were in a normal mental state, 4 had a GCS below 8, but these patients all
reported using a central nervous system depressant, most commonly GHB, with mephedrone. The patients also
reported polydrug use of a variety of compounds.[85]
Long-term effects
Almost nothing is known about the long-term effects of the drug due to the short history of its use.[84] BBC News
reported that one person who used the drug for 18 months became dependent on the drug, in the end using it twice a
week, had to be admitted to a psychiatric unit after he started experiencing hallucinations, agitation, excitability and
mania.[1] :13[86] Because of its similarity to cathinone, John Mann, has posited that mephedrone may cause
impotence with long-term use.[87]
Typical use and consumption
Mephedrone can come in the form of capsules, tablets or white powder that users may swallow, snort, inject, smoke
or use rectally.[1] :12[8] [9] It is sometimes sold mixed with methylone in a product called bubbles in the UK[88] and
also mixed with other cathinones including ethcathinone, butylone, fluoromethcathinone and methedrone.[1] :9 The
Guardian reported that some users compulsively redose, consuming their whole supply when they are only meant to
use a small dose[89] and there have been other similar reports of users craving mephedrone, suggesting that it may be
addictive.[1] :13[39] A survey conducted in late 2009 by the National Addiction Centre (UK) found 41.3% of readers
of Mixmag had used mephedrone in the last month, making it the fourth most popular drug amongst clubbers. Of
those, two thirds snorted the drug and the average dosage per session was 0.9 g; the length of sessions increased as
the dosage increased. Users who snorted the drug reported using more per session than those who took it orally
50
Mephedrone
(0.97 g compared to 0.74 g) and also reported using it more often (5 days per month compared to 3 days per
month).[8] An Irish study of people on a methadone treatment program for heroin addicts found that 29 out of 209
patients tested positive for mephedrone usage.[90] A study of users in Northern Ireland, found that they did not
perceive the fact that mephedrone was legal, with it being safe to use. This was contrary to another study in New
Zealand where users of benzylpiperazine thought that because it was legal it was safe.[14]
Harm reduction
The drugs advice charity Lifeline recommends that to reduce the potential harm caused by using mephedrone, users
should only use mephedrone occasionally (less than weekly), use less than 0.5 g per session, dose orally rather than
snorting the drug, and avoid mixing it with alcohol and other drugs. Users should also drink plenty of water whilst
taking the drug as it causes dehydration.[11]
Pharmacology
Very little is known about the pharmacology of mephedrone.[91]
Writing in the British Medical Journal, psychiatrists stated that given
its chemical structure, "mephedrone is likely to stimulate the release of,
and then inhibit the reuptake of monoamine neurotransmitters".[82] The
cathinone derivatives methcathinone and methylone, act in a similar
way to amphetamines mainly acting on catecholamine transporters so it
is expected that mephedrone also acts in this way. The actions of
amphetamines and cathinones are determined by the differences in how
they bind to noradrenalin, dopamine and serotonin transporters.[91]
Molecular modelling of mephedrone suggests it is more hydrophilic
than methyl-amphetamines which may account for the higher doses
required to achieve a similar effect, because mephedrone is less able to
cross the blood-brain barrier.[1] :12[92] Mephedrone has a chiral centre
The two enantiomers of mephedrone: the
and therefore exists in two forms, called enantiomers, it is thought that
potentially more potent S form is above the R
the S form is more potent than the R form, based on the fact that this
form
applies to cathinone.[91] Professor David Nutt, former chair of the
Advisory Council on the Misuse of Drugs (ACMD) in the UK has said
"people are better off taking ecstasy or amphetamines than those [drugs] we know nothing about" and "Who knows
what's in [mephedrone] when you buy it? We don't have a testing system. It could be very dangerous, we just don't
know. These chemicals have never been put into animals, let alone humans."[93] Les King, a former member of the
ACMD, has stated that mephedrone appears to be less potent than amphetamine and ecstasy but that any benefit
associated with this could be negated by users taking larger amounts. He also told the BBC "all we can say is
[mephedrone] is probably as harmful as ecstasy and amphetamines and wait until we have some better scientific
evidence to support that."[94]
Metabolism
Based on the analysis of rat and human urine by gas chromatography and mass spectrometry, mephedrone is thought
to be metabolised by three phase I pathways. It can be demethylated to the primary amine (producing compounds 2,
3 and 4) the ketone group can be reduced (producing 3) or the tolyl group can be oxidised (producing 5 and 6). It is
thought that 5 and 6 are further metabolised by conjugation to the glucuronide and sulfate derivatives. Knowledge of
the primary routes of metabolism should allow the intake of mephedrone to be confirmed by drug tests, as well as
more accurate determination of the cause of side effects and potential for toxicity.[95]
51
Mephedrone
52
[95]
Proposed scheme for the metabolism of mephedrone (1) based on the analysis of rat and human urine.
Toxicity
As of March 2010, there have been no reported studies on the potential neurotoxicity of mephedrone[82] nor is the
median lethal dose known.[8] In 2009, one case of sympathomimetic toxicity was reported in the UK after a person
took 0.2 g of mephedrone orally and then after this did not achieve the desired effect subcutaneously injected 3.8 g
mixed with water into his thighs. Shortly afterwards they "developed palpitations, 'blurred tunnel vision,' chest
pressure and sweating". The patient was treated with 1 mg of lorazepam and the sympathomimetic features
decreased and they were discharged within 6 hours of arrival.[96] The Swedish medical journal Läkartidningen
reported that mephedrone could theoretically cause the cardiovascular problems associated with the use of cocaine
and amphetamines and serotonin syndrome associated with the use of ecstasy and LSD.[97] One case of serotonin
syndrome has been reported, where the patient was already prescribed fluoxetine and olanzapine and then took 40
tablets containing mephedrone in one night. He was treated with lorazepam and discharged 15 hours after
admission.[98] Both enantiomers of methcathinone, which differs only in the lack of the methyl group on the aryl
ring when compared to mephedrone, have been shown to be toxic to rat dopamine neurons, and the S-enantiomer
was also toxic against serotonin neurons. Simon Gibbons and Mire Zloh of The School of Pharmacy, University of
London stated that based on the chemical similarities between methcathinone and mephedrone, "it is highly likely
that mephedrone will display neurotoxicity".[92] However, Brunt and colleagues stated that "extreme caution" should
be used when inferring the toxicity of mephedrone from methcathinone, noting that some of the toxicity associated
with methcathinone is due to manganese impurities related to its synthesis, rather than the compound itself. They
concluded that experimental research is needed to investigate the toxicity of mephedrone.[39] Doctors who treated a
15 year old female suffering from mephedrone intoxication suggested in The Lancet that like MDMA, mephedrone
may promote serotonin-mediated release of antidiuretic hormone resulting in hyponatraemia and an altered mental
state.[99] In another case, a 19 year old male was admitted to hospital suffering from inflammation of the heart, 20
hours after taking one gram of mephedrone. The doctors treating the patient stated it was caused by either a direct
toxic effect of mephedrone on the heart muscle, or by an immune response.[100] One case of acquired
methaemoglobinaemia, where a patient had "bluish lips and fingers", has also been reported, after they snorted one
gram of mephedrone. The patient started to recover after arriving at the hospital and it was not necessary to
Mephedrone
administer any medication.[101]
Deaths
Sweden
In 2008, an 18-year-old Swedish woman died in Stockholm after taking mephedrone. The tabloid newspaper Svenska
Dagbladet reported that the woman went into convulsions and turned blue in the face.[102] Doctors reported that she
was comatose and suffering from hyponatremia and severe hypokalemia; the woman died one and a half days after
the onset of symptoms. An autopsy showed severe brain swelling.[97] Mephedrone was scheduled to be classified as
a "dangerous substance" in Sweden even before the girl's death at Karolinska University Hospital on Sunday, 14
December, but the death brought more media attention to the drug. The possession of mephedrone became classified
as a criminal offence in Sweden on 15 December 2008.[102]
UK
In 2010, there were unconfirmed reports speculating about the role mephedrone has played in the deaths of several
young people in the UK. By July 2010, mephedrone had been alleged to be involved in 52 fatalities in the UK, but
detected in only 38 of these cases. Of the nine that coroners had finished investigating, two were caused directly by
mephedrone.[103] The first death reported to be caused by mephedrone use was that of 46 year old, Stirling Smith,
who had underlying health problems and repeatedly injected the drug.[104] A report in Forensic Science International
in August 2010 stated that mephedrone intoxication has been recorded as the cause of death in two cases in Scotland.
Post mortem samples showed the concentration of mephedrone in their blood was 22 mg/L in one case and 3.3 mg/L
in the other.[105] The death of a teenager in the UK in November 2009 was widely reported as being caused by
mephedrone, but a report by the coroner concluded that she died from natural causes.[50] In March 2010 the deaths of
two teenagers in Scunthorpe were widely reported by the media to be caused by mephedrone. Toxicology reports
showed that the teenagers had in fact not taken any mephedrone and that they in fact died as a result of consuming
alcohol and the heroin substitute methadone.[104] [106] According to Fiona Measham, a criminologist who is a
member of the ACMD, the reporting of the unconfirmed deaths by newspapers followed "the usual cycle of
‘exaggeration, distortion, inaccuracy and sensationalism'" associated with the reporting of recreational drug use.[35]
USA
Mephedrone has been implicated in the death of a 22 year old male, who had also injected black tar heroin.
Mephedrone was found in his blood at a concentration of 0.50 mg/L and in his urine at a concentration of 198 mg/L.
The blood concentration of morphine, a metabolite of heroin, was 0.06 mg/L.[107] For comparison, the average blood
morphine concentration resulting from deadly overdoses involving only heroin is around 0.34 mg/L.[108]
Chemistry
Appearance
Mephedrone is a white substance. It is sold most commonly as crystals or a powder, but also in the form of capsules
or pills.[24] [94] It can have a distinctive odour, reported to range from a synthetic fishy smell[109] to the smell of
vanilla and bleach, stale urine, electric circuit boards.[110]
53
Mephedrone
54
Synthesis
Mephedrone can be synthesised in several ways. The simplest method, due to the availability of the compounds,[1]
:17
is to add 4-methylpropiophenone dissolved in glacial acetic acid to bromine, creating an oil fraction of
4'-methyl-2-bromopropiophenone. The oil fraction can then be dissolved in dichloromethane (CH2Cl2) and drops of
the solution added to another solution of CH2Cl2 containing methylamine hydrochloride and triethylamine.
Hydrochloric acid (HCl) is then added and the aqueous layer is removed and turned alkaline using sodium hydroxide
before the amine is extracted using CH2Cl2. The CH2Cl2 is then evaporated using a vacuum, creating an oil which is
then dissolved in a non-aqueous ether. HCl gas is then bubbled through the mixture to produce
4-methylmethcathinone hydrochloride.[21] This method produces a mixture of both enantiomers and requires similar
knowledge to that required to synthesise amphetamines and MDMA.[1] :17
Mephedrone synthesis scheme from 4-methylpropiophenone
It can also be produced by oxidising the ephedrine analogue (4-methylephedrine) using potassium permanganate
dissolved in sulfuric acid. Because 4-methylephedrine can be obtained in a specific enantiomeric form it is possible
to produce mephedrone consisting of only one enantiomer. There is a danger associated with this method as it may
cause manganese poisoning if the product is not correctly purified.[1] :17
A stereospecific form of (S)-mephedrone could be prepared via Friedel–Crafts acylation. The first step in the
synthesis would react toluene and (S)-N-trifluoroacetylalanyl chloride in the presence of aluminium chloride, then
defluorinate the product in the presence of propyl alcohol and hydrochloric acid. This would produce
(S)-4-methylcathinone which could then be methylated to produce mephedrone.[91] [111]
Purity
One published study that analysed samples of mephedrone bought off the internet in the UK in 2010 found that it
was racemic (a mixture of both stereoisomers) and of high purity.[92] An unpublished study of six samples also
ordered off the internet in the UK in 2010 found that they contained very few organic impurities.[112] Four products
sold in Irish head shops that were tested in 2010 were found to contain between 82% and 14% mephedrone, with
some products containing benzocaine and caffeine.[113]
Mephedrone
Legal status
When mephedrone was rediscovered in 2003, it was
not specifically illegal to possess in any country, as its
use has increased many countries have passed
legislation making the possession, sale and
manufacturing of mephedrone illegal. It was first made
illegal in Israel, where it had been found in products
such as Neodoves pills, in January 2008.[12] After the
death of a young woman in Sweden in December 2008
was linked to the use of mephedrone, it was classified
as a hazardous substance a few days later, making it
illegal to sell in Sweden. In June 2009, it was classified
as a narcotic with the possession of 15 grams or more
resulting in a minimum of two years in prison - a
longer sentence, gram for gram than given for the
possession of cocaine or heroin.[114] [115] In December
A sample of mephedrone that was confiscated in Oregon, USA, 2009
[116]
2008, Denmark also made it illegal
and through the
Medicines Act of Finland it was made illegal to possess without a prescription.[117] In November 2009, it was
classified as a "narcotic or psychotropic" substance and added to the list of controlled substances in Estonia[118] and
made illegal to import into Guernsey along with other legal highs,[119] before being classified as a Class B drug in
April 2010.[120] It was classified as a Class C drug in Jersey in December 2009.[121]
In 2010, as its use became more prevalent, many countries passed legislation prohibiting mephedrone. It became
illegal in Croatia[122] and Germany[123] in January, followed by Romania[124] and the Isle of Man in February.[125] In
March 2010, it was classified as an unregulated medicine in the Netherlands, making the sale and distribution of it
illegal.[83] [126] The importation of mephedrone into the UK was banned on 29 March 2010.[127] The next day, the
ACMD in the UK published a report on the cathinones, including mephedrone, and recommended that they be
classifed as Class B drugs. On 7 April 2010 the Misuse of Drugs Act 1971 (Amendment) Order 2010 was passed by
parliament, making mephedrone and other substituted cathinones, Class B drugs from 16 April 2010.[128] [129] Prior
to the ban taking effect, mephedrone was not covered by the Misuse of Drugs Act 1971.[29] It was however an
offence under the Medicines Act to sell it for human consumption, so it was often sold as "plant food" or "bath salts"
although, as it has no use as these products, this too was possibly illegal under the Trade Descriptions Act 1968.[55]
[83] [84]
In May 2010 the Republic of Ireland made it illegal,[126] [130] [131] followed by Belgium,[132] Italy,[133]
Lithuania,[134] France[135] [136] and Norway[137] in June and Russia in July.[138] In August 2010, Austria[139] and
Poland[140] made mephedrone illegal and China announced that it would be illegal as of 1 September 2010.[30]
Mephedrone had been reported to be used in Singapore in February 2010,[141] but it was made illegal in November
2010.[142] In December 2010, following the advice of the EMCDDA, mephedrone was made illegal throughout the
EU, a move that Switzerland also made shortly afterwards.[143] [144] In countries, which have not already banned it,
such as the Netherlands, Greece, Spain and Portugal, they will need to change legislation to comply with the EU
ruling.[144] In Hungary, a government advisory body recommended that mephedrone should be made illegal in
August 2010, which was followed, making it illegal in January 2011.[145] [146]
In some countries, mephedrone is not specifically listed as illegal but is controlled under legislation that makes
compounds illegal if they are analogs of drugs already listed. In Australia during 2010 it was not specifically listed
as prohibited,[21] but the Australian Federal Police state that it is an analogue to methcathinone and therefore illegal.
In February 2010, 22 men were arrested in conjunction with importing mephedrone.[147] By January 2011, every
state in Australia, other than Victoria had listed it as a controlled drug.[148] In New Zealand it is not included in the
Misuse of Drugs Act 1975,[149] but is illegal as it is similar to controlled substances.[150] In Canada, mephedrone is
55
Mephedrone
not explicitly listed in any Schedule of the Controlled Drugs and Substances Act, "amphetamines, their salts,
derivatives, isomers and analogues and salts of derivatives, isomers and analogues" are included in Section I of
Schedule III of the act. Cathinone and methcathinone are listed in separate sections of Schedule III while
diethylpropion and pyrovalerone (also cathinones), are listed in separate sections of Schedule IV, each without
language to capture analogues, isomers, etc.[151] According to The Globe and Mail, mephedrone is considered a
controlled substance by Health Canada.[152] According to the Canadian Medical Association, mephedrone is grouped
with other amphetamines as Schedule III controlled substances.[153] There have been several media reports of the
Canadian police seizing mephedrone.[154] [155] [156] Mephedrone is also unscheduled in the United States. The Drug
Enforcement Administration state that as an analogue of methcathinone, possession of mephedrone can be controlled
by the Federal Analog Act, but according to the Los Angeles Times this only applies if it is sold for human
consumption.[157] [158] [159] Several cities and states have passed legislation to specifically list mephedrone as illegal,
but in most areas it is legal, so long as it is not sold for human consumption and therefore retailers describe it as 'bath
salts'.[159] Mephedrone is currently illegal in Michigan, North Dakota, Louisiana, and Florida.
References
[1] "Europol–EMCDDA Joint Report on a new psychoactive substance: 4-methylmethcathinone (mephedrone)" (http:/ / www. emcdda. europa.
eu/ attachements. cfm/ att_102496_EN_Europol-EMCDDA_Joint_Report_Mephedrone. pdf). European Monitoring Centre for Drugs and
Drug Addiction. 27 May 2010. . Retrieved 2011-01-29.
[2] http:/ / www. nlm. nih. gov/ cgi/ mesh/ 2009/ MB_cgi?term=1189805-46-6& rn=1
[3] http:/ / pubchem. ncbi. nlm. nih. gov/ summary/ summary. cgi?cid=29982893
[4] http:/ / www. chemspider. com/ Chemical-Structure. 21485694
[5] Meyer; Peters, M (2009). "Metabolism of the new designer drug mephedrone and toxicological detection of the beta keto designer drugs
mephedrone, butylone and methylone in urine" (http:/ / www. ata-journal. org/ articles/ ata/ pdf/ 2009/ 02/ ata2009s102. pdf) (PDF). Annales
de Toxicologie Analytique 21 (S1): 22. .
[6] http:/ / www. emolecules. com/ cgi-bin/ search?t=ex& q=Cc1ccc%28cc1%29C%28%3DO%29C%28C%29NC
[7] http:/ / pubchem. ncbi. nlm. nih. gov/ search/ ?smarts=Cc1ccc%28cc1%29C%28%3DO%29C%28C%29NC
[8] Winstock, A.; Mitcheson, L.; Deluca, P.; Davey, Z.; Corazza, O.; Schifano, F. (2010). "Mephedrone, new kid for the chop?". Addiction 106
(1): no. doi:10.1111/j.1360-0443.2010.03130.x. PMID 20735367.(subscription required)
[9] Matthews, A.; Bruno, R. (2010). "Mephedrone use among regular ecstasy consumers in Australia" (http:/ / www. webcitation. org/
5wZFYyEx7). Ecstasy and related drug trends bulletin. Archived from the original (http:/ / www. med. unsw. edu. au/ ndarcweb. nsf/
resources/ BulletinsEDRS2010/ $file/ EDRS+ December+ 2010. pdf) on 2011-02-18. .
[10] http:/ / en. wikipedia. org/ w/ index. php?& diff=cur& oldid=400834230
[11] "Mephedrone - Frequently asked questions" (http:/ / www. lifeline. org. uk/ docs/ Meph faq. pdf) (PDF). Lifeline publications. 2010. .
Retrieved 2010-09-17.
[12] Cumming, E. (22 April 2010). "Mephedrone: Chemistry lessons" (http:/ / www. telegraph. co. uk/ health/ 7614099/
Mephedrone-Chemistry-lessons. html). London: The Daily Telegraph. . Retrieved 2010-09-14.
[13] "Drugs crackdown hailed a success" (http:/ / news. bbc. co. uk/ 1/ hi/ scotland/ north_east/ 8555872. stm). BBC News. 8 March 2010. .
Retrieved 2010-03-31.
[14] McElrath, K.; O’Neill, C. (2011). "Experiences with mephedrone pre- and post-legislative controls: Perceptions of safety and sources of
supply". International Journal of Drug Policy 22 (2): 120–127. doi:10.1016/j.drugpo.2010.11.001. PMID 21242082.(subscription
required)
[15] Saem de Burnaga Sanchez, J. (1929). "Sur un homologue de l’éphédrine [On an analogue of ephedrine]" (in French). Bulletin de la Societé
Chimique de France 45: 284–286.
[16] Morris, H. (5 April 2010). "Hamilton’s Pharmacopeia. Mephedrone: the phantom menace" (http:/ / www. viceland. com/ int/ v17n6/ htdocs/
hamilton-s-pharmacopeia-455. php). Vice Magazine. . Retrieved 2010-07-04.
[17] Power, M. (January/February 2010). "How mephedrone shook the drug world - World Wired Web" (http:/ / www. drugscope. org. uk/
Resources/ Drugscope/ Documents/ PDF/ Good Practice/ DruglinkJanFeb10. pdf). Druglink. Drugscope. pp. 11–13. . Retrieved 2010-09-16.
[18] Power, M. (March/April 2009). "Mephedrone: the future of drug dealing?" (http:/ / www. drugscope. org. uk/ Resources/ Drugscope/
Documents/ PDF/ Good Practice/ Druglink_March_09_mephedrone. pdf). Druglink. Drugscope. pp. 6–9. . Retrieved 2010-09-16.
[19] Doward, J.; Shah, O. (26 April 2009). "There are many drugs that help people get out of their minds yet stay within the law - they're called
'legal highs'" (http:/ / www. guardian. co. uk/ politics/ 2009/ apr/ 26/ drugs-legal-substances-highs). The Observer. . Retrieved 2010-10-28.
[20] Bentur, Y.; Bloom-Krasik, A.; Raikhlin-Eisenkraft, B. (2008). "Illicit cathinone ("Hagigat") poisoning". Clinical toxicology 46 (3):
206–210. doi:10.1080/15563650701517574. PMID 17852166.(subscription required)
[21] Camilleri, A.; Johnston, M.; Brennan, M.; Davis, S.; Caldicott, D. (2010). "Chemical analysis of four capsules containing the controlled
substance analogues 4-methylmethcathinone, 2-fluoromethamphetamine, alpha-phthalimidopropiophenone and N-ethylcathinone". Forensic
56
Mephedrone
Science International 197 (1–3): 59–66. doi:10.1016/j.forsciint.2009.12.048. PMID 20074881.(subscription required)
[22] Davies, S.; Ramsey, J.; Archer, R. (November 2009). "Analytical profiles of Methcathinone Related Compounds" (http:/ / www. ltg. uk. net/
admin/ files/ Methcathinones. pdf). London Toxicology Group. . Retrieved 22 March 2010.
[23] "Psychonaut Web Mapping Project Newsletter" (http:/ / www. webcitation. org/ 5wOgF9upC). Psychonaut Web Mapping Project. June September 2009. Archived from the original (http:/ / 194. 83. 136. 209/ newsletters/ n2. php) on 2011-02-10. . Retrieved 2009-12-19.
[24] Roussel, O.; Perrin, M.; Herard, P.; Chevance, M.; Arpino, P. (2009). "La 4-méthyléphédrone sera-t-elle une "Ecstasy" du XXIème siècle [Is
4-methylephedrone, an "Ecstasy" of the twenty first century?]" (http:/ / www. ata-journal. org/ articles/ ata/ pdf/ 2009/ 05/ ata09028. pdf) (in
French). Annales de Toxicologie Analytique. doi:10.1051/ata/2009048. .
[25] Guppy, D. (18 June 2008). "Killer pills hit Cairns" (http:/ / www. cairns. com. au/ article/ 2008/ 06/ 18/ 4685_local-news. html).
Cairns.com.au. . Retrieved 2009-08-11.
[26] "Police warn of potentially fatal 'fake ecstasy'" (http:/ / www. webcitation. org/ 5wOj7ixX3). Abc.net.au. 17 June 2008. Archived from the
original (http:/ / www. abc. net. au/ news/ stories/ 2008/ 06/ 17/ 2277735. htm) on 2011-02-10. . Retrieved 2009-08-11.
[27] "EMCDDA 2008 Annual Report" (http:/ / www. emcdda. europa. eu/ attachements. cfm/ att_77265_EN_Annex 2_New psychoactive
substances reported 2008. pdf). European Monitoring Centre for Drugs and Drug Addiction. . Retrieved 2009-11-26.
[28] "DEA Microgram Bulletin - 4-Methylmethcathinone in Oregon" (http:/ / www. usdoj. gov/ dea/ programs/ forensicsci/ microgram/ mg0709/
mg0709. pdf). The Drug Enforcement Administration. July 2009. . Retrieved 2011-01-29.
[29] Devlin, K. (30 April 2009). "Psychiatrists call for 'legal high' drug 4-MMC to be banned" (http:/ / www. telegraph. co. uk/ science/
science-news/ 5244428/ Psychiatrists-call-for-legal-high-drug-4-MMC-to-be-banned. html). London: The Daily Telegraph. . Retrieved
2010-09-16.
[30] "Mephedrone regulated as psychotropic substances" (http:/ / eng. sfda. gov. cn/ cmsweb/ webportal/ W43879541/ A64031541. html). State
Food and Drug Administration, P.R. China. 2 August 2010. . Retrieved 2010-09-12.
[31] Gammell, C. (12 March 2009). "Legal online drugs providing real alternative to Class A substances" (http:/ / www. telegraph. co. uk/ health/
healthnews/ 4977750/ Legal-online-drugs-providing-real-alternative-to-Class-A-substances. html). London: The Daily Telegraph. . Retrieved
2010-09-16.
[32] Campbell, D. (17 January 2010). "Fears grow over safety of 'legal high' mephedrone" (http:/ / www. guardian. co. uk/ politics/ 2010/ jan/ 17/
drug-legal-methedrone-high). London: The Observer. . Retrieved 2010-03-19.
[33] Fleming, N. (18 March 2010). "Briefing: Should miaow-miaow be banned?" (http:/ / www. newscientist. com/ article/
dn18672-briefing-should-miaowmiaow-be-banned. html). New Scientist. . Retrieved 2010-09-16.
[34] (July 2010) Annex 1 to the Risk assessment report: Technical report on mephedrone (http:/ / www. ofdt. fr/ BDD/ publications/ docs/
rarOEDTmephAnn1. pdf). European Monitoring Centre for Drugs and Drug Addiction. (Report). Retrieved 2010-09-24.
[35] Measham, F.; Moore, K.; Newcombe, R.; Smith, Z. (12 March 2010). "Tweaking, bombing, dabbing and stockpiling: the emergence of
mephedrone and the perversity of prohibition". Drugs and Alcohol Today 10 (1): 14–21. doi:10.5042/daat.2010.0123.(subscription
required)
[36] (October 2010) Drug Treatment in 2009-10 (http:/ / www. nta. nhs. uk/ uploads/ nta_annualreport_0910. pdf). National Treatment Agency
for Substance Misuse. (Report). Retrieved 2010-10-27.
[37] Fleming, N. (29 March 2010). "Miaow-miaow on trial: Truth or trumped-up charges?" (http:/ / www. newscientist. com/ article/
dn18712-miaowmiaow-on-trial-truth-or-trumpedup-charges. html?full=true). New Scientist. . Retrieved 2010-04-01.
[38] Reed, J. (20 June 2010). "Newsbeat - Ecstasy 'disappearing' from British clubs" (http:/ / www. bbc. co. uk/ newsbeat/ 10353130). BBC. .
Retrieved 2010-08-30.
[39] Brunt, T.; Poortman, A.; Niesink, R.; Van Den Brink, W. (2010). "Instability of the ecstasy market and a new kid on the block:
mephedrone". Journal of psychopharmacology. doi:10.1177/0269881110378370. PMID 20826554.(subscription required)
[40] "The Misuse of Drugs Act 1971 (Amendment) Order 2009" (http:/ / www. legislation. gov. uk/ ukdsi/ 2009/ 9780111486610/ contents). UK
government. October 2009. . Retrieved 2011-01-31.
[41] Silverman, J. (2010). "Addicted to distortion: the media and UK drugs policy". Safer Communities 9 (4): 26–31.
doi:10.5042/sc.2010.0582.(subscription required)
[42] James, D.; Adams, R.; Spears, R.; Cooper, G.; Lupton, D.; Thompson, J.; Thomas, S.; on behalf of the National Poisons Information Service
(2010). "Clinical characteristics of mephedrone toxicity reported to the UK National Poisons Information Service". Emergency medicine
journal. doi:10.1136/emj.2010.096636. PMID 20798084.
[43] "Inquiries over mephedrone effects" (http:/ / www. webcitation. org/ 5wOgKLEum). Press Association. 4 October 2010. Archived from the
original (http:/ / www. pressassociation. com/ component/ pafeeds/ 2010/ 10/ 04/ inquiries_over_mephedrone_effects) on 2011-02-10. .
Retrieved 2010-10-27.
[44] Chaffey, N. (2010). "Plant Cuttings - When is a plant food not a plant food?". Annals of Botany 105 (6): v–viii. doi:10.1093/aob/mcq112.
[45] Chaffey, N. (2010). "Plant Cuttings - Clarification". Annals of Botany 106 (4): iii. doi:10.1093/aob/mcq194.
[46] Private Eye, "Street of Shame", No. 1259, 2–15 April 2010, p. 6: Way back in January 2009, not long after mephedrone first began to be
sold online, members of the web forum attached to the now-defunct "headshop" Champagne Legals discussed what brand name they might
attach to the new product, which has the chemical identity dimethylmethcathinone or MM-Cat.
"What shall we call this drug? It's called MM-CAT, so why not Miaow?" suggested one. The name did not catch on
... But on 1 November 2009, someone did add the name "Meow" to the Wikipedia entry for Mephedrone at the head
57
Mephedrone
of a list of "street names."
Three weeks later ... the Sun declared the arrival of a "new party favourite called 'meow meow'" and the world went
cat-call crazy.
Among a host of recent headlines the Sunday Times has reported on "the rise of Meow", the Times has heralded
"Meow Meow arrests", the Sun shrieked about a "Harman snub for Meow Meow Ban" and the Daily Telegraph took
a long hard look at the "Meow Meow Menace in Europe."
"No one ever called it Meow seriously till the papers picked up on the Wikipedia entry," one drugs expert tells the
Eye.
[47] Soodin, V. (26 November 2009). "'Meow meow' drug teen ripped his scrotum off" (http:/ / www. webcitation. org/ 5wOf8HlGi). The Sun.
Archived from the original (http:/ / www. thesun. co. uk/ sol/ homepage/ news/ 2747979/ Lad-ripped-his-scrotum-off. html) on 2011-02-10. .
Retrieved 2010-09-15.
[48] Davey, Z.; Corazza, O.; Schifano, F.; Deluca, P. (2010). "Mass-information: mephedrone, myths, and the new generation of legal highs".
Drugs and Alcohol Today 10 (3): 24. doi:10.5042/daat.2010.0467.(subscription required)
[49] Greenslade, R. (2 December 2010). "New guide aims to bring sense to the media coverage of drugs" (http:/ / www. guardian. co. uk/ media/
greenslade/ 2010/ dec/ 02/ drugs-newspapers). The Guardian. . Retrieved 2010-12-19.
[50] Fleming, N. (5 April 2010). "Mephedrone: the anatomy of a media drug scare" (http:/ / www. guardian. co. uk/ media/ 2010/ apr/ 05/
mephedrone-drug-media-scare-newspapers). London: The Guardian. . Retrieved 2010-09-20.
[51] Anonymous editorial (2010). "A collapse in integrity of scientific advice in the UK". The Lancet 375 (9723): 1319–1319.
doi:10.1016/S0140-6736(10)60556-9.
[52] Silverman, J. (2010). "Addicted to getting drugs wrong" (http:/ / www. webcitation. org/ 5w9C0MRCS). British Journalism Review 21 (4):
31–36. doi:10.1177/0956474810393603. Archived from the original (http:/ / bjr. org. uk/ data/ 2010/ no4_silverman) on 2011-01-31. .
[53] Dargan, P. I.; Albert, S.; Wood, D. M. (2010). "Mephedrone use and associated adverse effects in school and college/university students
before the UK legislation change". QJM 103 (11): 875–9. doi:10.1093/qjmed/hcq134. PMID 20675396.
[54] "Mephedrone to be made Class B drug 'within weeks'" (http:/ / news. bbc. co. uk/ 1/ hi/ uk/ 8592103. stm). BBC News. 29 March 2010. .
Retrieved 2010-03-31.
[55] "Consideration of the cathinones" (http:/ / www. webcitation. org/ 5wOj4Dvbn). Advisory Council on the Misuse of Drugs. 31 March 2010.
p. 25. Archived from the original (http:/ / www. homeoffice. gov. uk/ publications/ drugs/ acmd1/ acmd-cathinodes-report-2010?view=Binary)
on 2011-02-10. . Retrieved 2011-02-10.
[56] Kmietowicz, Z. (2010). "Home secretary bans mephedrone after taking advice from depleted council". British Medical Journal 340: c1784.
doi:10.1136/bmj.c1784. PMID 20356967.
[57] Taylor, P. (2010-03-29). "Drug adviser Dr Polly Taylor's full resignation letter" (http:/ / news. bbc. co. uk/ 1/ hi/ uk/ 8592157. stm). BBC
News. . Retrieved 2010-09-15.
[58] "Resignation 'threatens drug ban'" (http:/ / news. bbc. co. uk/ 1/ hi/ uk/ 8592103. stm). BBC News. 29 March 2010. . Retrieved 2010-03-29.
[59] "Government adviser Eric Carlin resigns over mephedrone" (http:/ / news. bbc. co. uk/ 1/ hi/ uk/ 8601315. stm). BBC News. 2 April 2010. .
Retrieved 2010-04-02.
[60] Rolles, S. (18 March 2010). "Mephredone and the ACMD: lessons from BZP and New Zealand's "Class D' experiment?" (http:/ / www.
webcitation. org/ 5wOfibOWm). Transform Drug Policy Foundation. Archived from the original (http:/ / transform-drugs. blogspot. com/
2010/ 03/ mephedrone-and-acmd-lessons-from-bzp. html) on 2011-02-10. . Retrieved 13 April 2010.
[61] Doward, J. (4 April 2010). "Mephedrone row grows as seventh member of drugs panel" (http:/ / www. guardian. co. uk/ society/ 2010/ apr/
04/ eric-carlin-mephedrone-classification). London: The Observer. . Retrieved 2010-09-15.
[62] Carlin, E. (2 April 2010). "Eric Carlin's letter of resignation from the ACMD" (http:/ / news. bbc. co. uk/ 1/ hi/ uk/ 8600929. stm). BBC
News. . Retrieved 2010-04-13.
[63] Eastwood, N. (June 2010). "Legal Eye - Mephedrone becomes a class B drug" (http:/ / pierprofessional. metapress. com/ content/
f480l21302614782/ ?p=3ab785d1044847afa8050a4f2731eb21& pi=1). Drugs and Alcohol Today 10 (2): 6–9. doi:10.5042/daat.2010.0251.
.(subscription required)
[64] "Decision to outlaw mephedrone drug not connected to teen deaths" (http:/ / www. webcitation. org/ 5wOfLlobW). The Scunthorpe
Telegraph. 27 January 2011. Archived from the original (http:/ / www. thisisscunthorpe. co. uk/ news/
Decision-outlaw-drug-connected-deaths/ article-3149995-detail/ article. html) on 2011-02-10. . Retrieved 2011-02-10.
[65] Mann, J. (April 2010). "Can we halt the flow of new designer drugs?" (http:/ / www. rsc. org/ chemistryworld/ Issues/ 2010/ April/
CanWeHaltTheFlowOfNewDesignerDrugs. asp). Chemistry World. . Retrieved 2010-09-16.
[66] Sare, J. (September/October 2010). "An unchartered course" (http:/ / www. drugscope. org. uk/ Resources/ Drugscope/ Documents/ PDF/
Publications/ DruglinkSept-Oct2010. pdf). Druglink. Drugscope. p. 5. . Retrieved 2011-01-29.
[67] Stratton, E. (8 December 2010). "Proposals for banning drugs are more draconian than they seem" (http:/ / www. guardian. co. uk/ science/
political-science/ 2010/ dec/ 08/ proposals-banning-drugs). The Guardian. . Retrieved 2010-12-19.
[68] Van Noorden, R. (2010-12-06). "Science advice not mandatory on drugs council, proposes UK government" (http:/ / www. webcitation. org/
5wOifkrhN). Nature News. Archived from the original (http:/ / blogs. nature. com/ news/ thegreatbeyond/ 2010/ 12/
58
Mephedrone
science_advice_not_mandatory_o_1. html) on 2011-02-10. . Retrieved 2010-12-20.
[69] "Mephedrone: harm reduction advice" (http:/ / www. drugscience. org. uk/ mephedroneadvice. html). Drugscience.org.uk. Independent
Scientific Committee on Drugs. 2010. . Retrieved 2010-09-15.
[70] Daly, M. (September/October 2010). "Booze, bans and bite-size bags" (http:/ / www. drugscope. org. uk/ Resources/ Drugscope/
Documents/ PDF/ Publications/ DruglinkSept-Oct2010. pdf). Druglink. Drugscope. pp. 6–9. . Retrieved 2011-01-29.
[71] Winstock, A.; Mitcheson, L.; Marsden, J. (November 2010). "Mephedrone: still available and twice the price". The Lancet 376 (9752):
1537. doi:10.1016/S0140-6736(10)62021-1.(subscription required)
[72] "Clubbers 'still using' banned drug mephedrone" (http:/ / www. bbc. co. uk/ newsbeat/ 12389389). BBC News. 8 February 2011. . Retrieved
2011-02-10.
[73] Garnett, N. (9 February 2011). "Mephedrone freely available on the internet despite ban" (http:/ / www. bbc. co. uk/ news/ uk-12389321).
BBC Radio 5 Live. . Retrieved 2011-02-17.
[74] Laurance (24 November 2010). "Mephedrone ban blamed for rise in cocaine deaths" (http:/ / www. independent. co. uk/ life-style/
health-and-families/ health-news/ mephedrone-ban-blamed-for-rise-in-cocaine-deaths-2142097. html). The Independent. . Retrieved
2010-12-20.
[75] Bird, S. (22 November 2010). "Banned drug may have saved lives, not cost them" (http:/ / www. straightstatistics. org/ article/
banned-drug-may-have-saved-lives-not-cost-them). Straight Statistics. . Retrieved 2010-12-20.
[76] "NRG-1 'legal high' drug is banned" (http:/ / news. bbc. co. uk/ 1/ hi/ uk/ 10602398. stm). BBC News. 12 July 2010. . Retrieved 2010-08-23.
[77] M., S.; Power (17 August 2010). "Ivory Wave drug implicated in death of 24-year-old man" (http:/ / www. guardian. co. uk/ society/ 2010/
aug/ 17/ ivory-wave-drug-alleged-death). London: The Guardian. . Retrieved 2010-08-23.
[78] Brandt, S.; Sumnall, H.; Measham, F.; Cole, J. (2010). "The confusing case of NRG-1". British Medical Journal 341: c3564.
doi:10.1136/bmj.c3564. PMID 20605894.(subscription required)
[79] "Students test mephedrone drug" (http:/ / news. bbc. co. uk/ 1/ hi/ education/ 8582472. stm). BBC News. 23 March 2010. . Retrieved 23
March 2010.
[80] "Mephedrone may be banned, chief drug adviser indicates" (http:/ / news. bbc. co. uk/ 1/ hi/ uk/ 8582999. stm). BBC News. 23 March 2010.
. Retrieved 2010-03-23.
[81] Faulkner, A. (16 August 2010). "Mephedrone drugs study scrapped by Liverpool university" (http:/ / www. clickliverpool. com/ news/
national-news/ 1210293-mephedrone-drugs-study-scrapped-by-a-liverpool-university. html). Click Liverpool. . Retrieved 2010-08-30.
[82] Winstock, A.; Marsden, J.; Mitcheson, L. (23 March 2010). "What should be done about mephedrone?". British Medical Journal 340:
c1605. doi:10.1136/bmj.c1605. PMID 20332508.(subscription required)
[83] "Police warning over 'bubble' drug" (http:/ / news. bbc. co. uk/ 1/ hi/ england/ tees/ 8370130. stm). BBC News. 20 November 2009. .
Retrieved 2009-11-27.
[84] Reed, J. (13 January 2010). "Clubbers are 'turning to new legal high mephedrone'" (http:/ / news. bbc. co. uk/ newsbeat/ hi/ health/
newsid_10000000/ newsid_10004300/ 10004366. stm). BBC News. . Retrieved 2010-07-04.
[85] Wood, D.; Greene, S.; Dargan, P. (2010). "Clinical pattern of toxicity associated with the novel synthetic cathinone mephedrone".
Emergency Medicine Journal 28 (4): 280–282. doi:10.1136/emj.2010.092288. PMID 20581379.(subscription required)
[86] "Call for ban on 'legal high' drug" (http:/ / news. bbc. co. uk/ 1/ hi/ health/ 8023451. stm). BBC News. 30 April 2009. . Retrieved
2010-09-20.
[87] "Mephedrone can cause impotence, warns expert" (http:/ / www. telegraph. co. uk/ health/ healthnews/ 7545261/
Mephedrone-can-cause-impotence-warns-expert. html). London: The Daily Telegraph. 1 April 2010. . Retrieved 2010-07-04.
[88] "A joined-up approach to drugs is needed now" (http:/ / www. heraldscotland. com/ comment/ herald-view/
a-joined-up-approach-to-drugs-is-needed-now-1. 1014820). Herald Scotland. 2010-03-21. . Retrieved 2010-09-15.
[89] Campbell, Duncan (12 March 2009). "Online sales of legal alternatives to class A drugs raise safety fears" (http:/ / www. guardian. co. uk/
society/ 2009/ mar/ 12/ online-legal-drugs-stimulants). London: The Guardian. . Retrieved 2010-09-20.
[90] McNamara, S.; Stokes, S.; Coleman, N. (2009). "Head Shop Compound abuse amongst attendees of The Drug Treatment Centre Board"
(http:/ / www. webcitation. org/ 5wOixdFRM). The Irish Medical Journal 102 (5). Archived from the original (http:/ / www. imj. ie/
ViewArticleDetails. aspx?ArticleID=5909) on 2011-02-10. .
[91] Schifano, F.; Albanese, A.; Fergus, S.; Stair, J.; Deluca, P.; Corazza, O.; Davey, Z.; Corker, J. et al. (2010). "Mephedrone
(4-methylmethcathinone; 'meow meow'): chemical, pharmacological and clinical issues". Psychopharmacology 214 (3): 593–602.
doi:10.1007/s00213-010-2070-x. PMID 21072502.(subscription required)
[92] Gibbons, S.; Zloh, M. (2010). "An analysis of the ‘legal high’ mephedrone". Bioorganic & Medicinal Chemistry Letters 20 (14): 4135–4139.
doi:10.1016/j.bmcl.2010.05.065. PMID 20542690.(subscription required)
[93] Saner, E. (5 December 2009). "Mephedrone and the problem with 'legal highs'" (http:/ / www. guardian. co. uk/ society/ 2009/ dec/ 05/
mephedrone-problem-legal-highs). London: The Guardian. . Retrieved 2010-09-20.
[94] Reed, J. (13 January 2010). "What is legal high mephedrone?" (http:/ / news. bbc. co. uk/ newsbeat/ hi/ health/ newsid_10000000/
newsid_10004300/ 10004383. stm). BBC Newsbeat. . Retrieved 2010-09-20.
[95] Meyer, M.; Wilhelm, J.; Peters, F.; Maurer, H. (2010). "Beta-keto amphetamines: studies on the metabolism of the designer drug
mephedrone and toxicological detection of mephedrone, butylone, and methylone in urine using gas chromatography–mass spectrometry".
Analytical and Bioanalytical Chemistry 397 (3): 1225–1233. doi:10.1007/s00216-010-3636-5. PMID 20333362.(subscription required)
59
Mephedrone
[96] Wood, D. M.; Davies, S.; Puchnarewicz, M.; Button, J.; Archer, R.; Ovaska, H.; Ramsey, J.; Lee, T. et al. (2010). "Recreational Use of
Mephedrone (4-Methylmethcathinone, 4-MMC) with Associated Sympathomimetic Toxicity". Journal of Medical Toxicology 6 (3): 327–30.
doi:10.1007/s13181-010-0018-5. PMID 20358417.(subscription required)
[97] Gustavsson, D.; Escher, C. (20 October 2009). "Mefedron – Internetdrog som tycks ha kommit för att stanna [Mephedrone - Internet drug
that seems to have come to stay]" (http:/ / www. lakartidningen. se/ 07engine. php?articleId=12986) (in Swedish). Lakartidningen. .
[98] Garrett, G.; Sweeney, M. (2010). "The serotonin syndrome as a result of mephedrone toxicity". British Medical Journal Case Reports 2010:
bcr0420102925. doi:10.1136/bcr.04.2010.2925.(subscription required)
[99] Sammler, E.; Foley, P.; Lauder, G.; Wilson, S.; Goudie, A.; O'Riordan, J. (2010). "A harmless high?". The Lancet 376 (9742): 742.
doi:10.1016/S0140-6736(10)60891-4.(subscription required)
[100] Nicholson, P.; Quinn, M.; Dodd, J. (2010). "Headshop heartache: acute mephedrone 'meow' myocarditis". British Medical Journal: Heart
96 (24): 2051–2. doi:10.1136/hrt.2010.209338. PMID 21062771.(subscription required)
[101] Ahmed, N.; Hoy, B.; McInerney, J. (2010). "Methaemoglobinaemia due to mephedrone ('snow')". British Medical Journal Case Reports
2010: bcr0420102879. doi:10.1136/bcr.04.2010.2879.(subscription required)
[102] "Teenager dies of 'net drug' overdose" (http:/ / www. thelocal. se/ 16366/ 20081215/ ). The Local. 15 December 2008. . Retrieved
2010-09-20.
[103] Ghodse, H.; Corkery, J.; Ahmed, K.; Naidoo, V.; Oyefeso and, A.; Schifano, F. (July 2010). "Drug-related deaths in the UK: Annual
Report 2010" (http:/ / www. webcitation. org/ 5vR9zRdew) (PDF). International Centre for Drug Policy, St George's University of London. p.
77. Archived from the original (http:/ / www. sgul. ac. uk/ about-st-georges/ divisions/ faculty-of-medicine-and-biomedical-sciences/
mental-health/ icdp/ website-pdfs/ np-SAD 11th annual report Final. pdf) on 2010-01-02. . Retrieved 2010-01-02.
[104] "Teenagers' deaths 'not caused by mephedrone'" (http:/ / news. bbc. co. uk/ 1/ hi/ uk/ 10184803. stm). BBC News. 28 May 2010. .
Retrieved 2010-09-20.
[105] Torrance, H.; Cooper, G. (2010). "The detection of mephedrone (4-methylmethcathinone) in 4 fatalities in Scotland". Forensic Science
International 202 (1): e62–e63. doi:10.1016/j.forsciint.2010.07.014. PMID 20685050.
[106] "Scunthorpe community 'awash with methadone'" (http:/ / www. bbc. co. uk/ news/ uk-england-humber-12275383). BBC News. 25
January 2011. . Retrieved 2011-02-10.
[107] Past, A.; Vorce, S.; Levine, B; Past, MR (2010). "Case report: multiple-drug toxicity caused by the coadministration of
4-methylmethcathinone (mephedrone) and heroin" (http:/ / www. jatox. com/ index. php/ Articles/
Case-Report-Multiple-Drug-Toxicity-Caused-by-the-Coadministration-of-4-Methylmethcathinone-Mephedrone-and-Heroin. html). Journal of
Analytical Toxicology 34 (3): 162–168. PMID 20406541. .(subscription required)
[108] Shane Darke, Deborah Zador (1996). "Fatal heroin 'overdose': a review" (http:/ / www. lindesmith. org/ library/ darke2. cfm). Addiction 91
(12): 1765–1772. doi:10.1046/j.1360-0443.1996.911217652.x. PMID 8997759. .
[109] Power, M.; Parry, S. (24 April 2010). "The Chinese laboratories where scientists are already at work on the new 'meow meow'" (http:/ /
www. webcitation. org/ 5wOgMsftf). Daily Mail (London). Archived from the original (http:/ / www. dailymail. co. uk/ home/ moslive/
article-1267582/ The-Chinese-laboratories-scientists-work-new-meow-meow. html) on 2011-02-10. . Retrieved 2010-09-13.
[110] Psychonaut WebMapping Research Group (March 2010). "Mephedrone Report" (http:/ / www. webcitation. org/ 5wOgBGB37) (PDF).
Institute of Psychiatry, King's College London. Archived from the original (http:/ / 194. 83. 136. 209/ documents/ reports/ Mephedrone. pdf)
on 2011-02-10. . Retrieved 2011-01-31.
[111] Osorio-Olivares, M. (2003). "A two-step method for the preparation of homochiral cathinones". Tetrahedron: Asymmetry 14 (11):
1473–1477. doi:10.1016/S0957-4166(03)00317-3.(subscription required)
[112] "Risks of banned drug mephedrone revealed in new research" (http:/ / www. sciencedaily. com/ releases/ 2010/ 07/ 100714104231. htm).
Science Daily. 15 July 2010. . Retrieved 2011-02-10.
[113] Kavanagh, P.; McNamara, S.; Angelov, D.; McDermott, S.; Mullan, D.; Ryder, S. (March 2010). "The Characterization of ‘Legal Highs’
Available from Head Shops in Dublin" (http:/ / addictionireland. com/ _fileupload/ publications/ Legal_Highs_Poster. pdf). The Drug
Treatment Centre Board. . Retrieved 2011-02-17.
[114] "Svensk författningssamling Förordning om ändring i förordningen (1999:58) om förbud mot vissa hälsofarliga varor [Swedish Code of
Statutes. Regulation amending the Ordinance (1999:58) banning certain hazardous goods]" (http:/ / www. webcitation. org/ 5wOfbf5sB) (in
Swedish). 25 November 2008. Archived from the original (http:/ / 62. 95. 69. 3/ SFSDOC/ 08/ 080908. PDF) on 2011-02-10. . Retrieved
2011-01-31.
[115] Stigson, G. (9 June 2010). "15 gram narkotika ger två års fängelse [15 grams of drugs means two years in prison]" (http:/ / www.
webcitation. org/ 5wOfFjNlN) (in Swedish). Dala Demokraten. Archived from the original (http:/ / www. dalademokraten. se/ sida/ id/
124262) on 2011-02-10. . Retrieved 2010-09-20.
[116] (2009) 2009 National Report (2008 data) To the EMCDDA by the Reitox National Focal Point - Denmark (http:/ / www. emcdda. europa.
eu/ attachements. cfm/ att_112008_EN_NR_2009_DK. pdf). (Report). Retrieved 2010-09-14.
[117] "Rapujuhlat saaristossa [Crayfish parties in the archipelago]" (http:/ / www. city. fi/ artikkeli/ Rapujuhlat+ saaristossa+ / 2783/ ) (in
Finnish). City Magazine. September 2009. . Retrieved 2010-09-14.
[118] Estonian Ministry of Social Affairs (2009-12-08). "Sotsiaalministri 27.11.2009 määrus number 87 [Minister of Social Affairs regulation
number 87]" (http:/ / www. webcitation. org/ 5suaZrvK9) (in Estonian). Archived from the original (http:/ / www. estlex. ee/ tasuta/ ?id=8&
aktid=118262& fd=1& asutus=& grupp=3& leht=2) on 2010-09-21. . Retrieved 2010-09-21.
60
Mephedrone
[119] "Guernsey mephedrone ban 'only weeks away'" (http:/ / news. bbc. co. uk/ 1/ hi/ world/ europe/ guernsey/ 8604784. stm). BBC News. 6
April 2010. . Retrieved 2010-09-14.
[120] "Guernsey makes Mephedrone class B drug" (http:/ / news. bbc. co. uk/ 1/ hi/ world/ europe/ guernsey/ 8618651. stm). BBC News. 13
April 2010. . Retrieved 2010-09-14.
[121] Campbell, A. (4 March 2010). "Call to ban legal high mephedrone test change" (http:/ / news. bbc. co. uk/ newsbeat/ hi/ health/
newsid_10050000/ newsid_10059100/ 10059133. stm). BBC Newsbeat. . Retrieved 17 March 2010.
[122] "Dopuna popisa opojnih droga, psihotropnih tvari i biljaka iz kojih se može dobiti opojna droga te tvari koje se mogu uporabiti za izradu
opojnih droga [List of narcotic drugs, psychotropic substances and plants from which narcotic drugs and substances that may be used to
manufacture drugs can be obtained]" (http:/ / narodne-novine. nn. hr/ clanci/ sluzbeni/ 2010_01_2_12. html) (in Croatian). Croatian Ministry
of Health and Social Welfare. 28 December 2009. . Retrieved 2010-07-04.
[123] "Vierundzwanzigste Verordnung zur Änderung betäubungsmittelrechtlicher Vorschriften [24th Amendment of narcotics legislation]"
(http:/ / www. buzer. de/ gesetz/ 9164/ index. htm) (in German). Selected laws in Germany - Buzer.de. 22 January 2010. . Retrieved
2010-07-04.
[124] Romanian Health Ministry (10 February 2010). "Comunicat de presă Ministerul Sănătăţii a stabilit lista cu plante şi substanţe cu proprietăţi
psihoactive care vor fi interzise, după ce s-au dovedit a fi periculoase pentru sănătate [Press release: The Health Ministry has established a list
of plants and other substances with psychoactive properties that will be banned, after it has been proven that they are dangerous to health]"
(http:/ / www. webcitation. org/ 5wOg4I4KV) (in Romanian). Romanian Health Ministry. Archived from the original (http:/ / www. ms. ro/
index. php?pag=62& id=8095& pg=1) on 2011-02-10. . Retrieved 2010-09-21.
[125] "Now illegal to import drug 'plant food' to Isle of Man" (http:/ / www. iomtoday. co. im/ news/ Now-illegal-to-import-drug. 6103144. jp).
Isle of Man Today. 25 February 2010. . Retrieved 2010-07-04.
[126] Kelly, F. (3 March 2010). "Head shop substances to be banned" (http:/ / www. independent. ie/ national-news/
head-shop-substances-to-be-banned-2086255. html). Irish Independent. . Retrieved 2010-09-21.
[127] Ilston, G. (1 April 2010). "Mephedrone to be classified a Class B drug" (http:/ / www. webcitation. org/ 5wOgQBTy2). Police Professional.
Archived from the original (http:/ / www. policeprofessional. com/ news. aspx?id=10163) on 2011-02-10. . Retrieved 2010-09-21.
[128] "BBC - Democracy Live - MPs move to ban mephedrone" (http:/ / news. bbc. co. uk/ democracylive/ hi/ house_of_commons/
newsid_8605000/ 8605939. stm). BBC News. 7 April 2010. . Retrieved 2010-04-08.
[129] "The Misuse of Drugs (Amendment) (England, Wales and Scotland) Regulations 2010 No. 1144" (http:/ / www. webcitation. org/
5wOiOGPha). Office of Public Sector Information. 16 April 2010. Archived from the original (http:/ / www. legislation. gov. uk/ uksi/ 2010/
1144/ made) on 2011-02-10. . Retrieved 2010-04-08.
[130] "Minister for Health and Children announces immediate criminal ban on list of head shop products" (http:/ / www. dohc. ie/ press/ releases/
2010/ 20100511. html). Irish Department of Health and Children. 11 May 2010. . Retrieved 2010-08-22.
[131] Gartland, F. (6 November 2009). "Irish youth are fourth highest cocaine users in Europe" (http:/ / www. webcitation. org/ 5nZOG07Dt).
The Irish Times. Archived from the original (http:/ / www. irishtimes. com/ newspaper/ ireland/ 2009/ 1106/ 1224258193510. html) on
2010-02-15. . Retrieved 2010-09-21.
[132] "Arrêté royal du 13 Juin 2010 portant modification de l'arrêté royal du 22 janvier 1998 réglementant certaines substances psychotropes, et
relatif à la réduction des risques et à l'avis thérapeutique [Royal Decree of 13 June 2010 amending the Royal Decree of 22 January 1998
regulating certain psychotropic substances, therapeutic advice and on reducing risk]" (http:/ / www. ejustice. just. fgov. be/ cgi/ article_body.
pl?numac=2010018212& caller=list& pub_date=2010-06-21& language=fr) (in French). Belgian Department of Justice. 13 June 2010. .
Retrieved 2010-07-05.
[133] "Tabelle sostanze stupefacenti e psicotrope [Table of drugs and psychotropics]" (http:/ / www. salute. gov. it/ medicinaliSostanze/
paginaInternaMedicinaliSostanze. jsp?id=7& menu=strumenti) (in Italian). Italian Ministry of Health. 10 June 2010. . Retrieved 2011-01-02.
[134] Ministry of Health of the Republic of Lithuania (18 June 2010). "2000 m. sausio 6 d. įsakymo nr. 5 "dėl narkotinių ir psichotropinių
medžiagų sąrašų patvirtinimo" pakeitimo [6 January 2000 Order no. 5 of narcotic drugs and psychotropic substances list]" (http:/ / www.
webcitation. org/ 5suaM3hwb) (in Lithuanian). Archived from the original (http:/ / www3. lrs. lt/ pls/ inter3/ dokpaieska.
showdoc_l?p_id=375830) on 2010-09-21. . Retrieved 2010-09-21.
[135] AFP (11 June 2010). "Classement comme stupéfiant de la méphédrone, dérivée du khat [Classification of mephedrone from khat as a
narcotic]" (http:/ / www. france24. com/ fr/ 20100611-classement-comme-stupefiant-mephedrone-derivee-khat) (in French). france24.com. .
Retrieved 2010-07-04.
[136] "La méphédrone classée comme stupéfiant [Mephedrone classified as a narcotic]" (http:/ / www. webcitation. org/ 5tlhcDpGD) (in French).
French Ministry of Health and Sports. 11 June 2010. Archived from the original (http:/ / www. sante-sports. gouv. fr/
la-mephedrone-classee-comme-stupefiant. html) on 2010-10-26. . Retrieved 2010-10-26.
[137] "Ti nye stoffer på narkotikalisten [Ten new compounds for drug list]" (http:/ / www. webcitation. org/ 5wOijcYrp) (in Norwegian).
Norwegian Broadcasting Corporation (NRK). 11 June 2010. Archived from the original (http:/ / www. nrk. no/ nyheter/ norge/ 1. 7163768) on
2011-02-10. . Retrieved 2010-08-31.
[138] Government of the Russian Federation (29 July 2010). "Постановление от 29 июля 2010 г. №578 [Decree of 29 July 2010 number 578]"
(http:/ / www. webcitation. org/ 5wOg1EyxH) (in Russian). Archived from the original (http:/ / government. ru/ gov/ results/ 11583/ ) on
2011-02-10. . Retrieved 2010-11-19.
[139] "Mode-Droge Mephedron in Österreich verboten [Fashionable drug mephedrone illegal in Austria]" (http:/ / www. webcitation. org/
5wOj9xom7) (in German). Die Presse. 22 August 2010. Archived from the original (http:/ / diepresse. com/ home/ panorama/ 589205/ index.
61
Mephedrone
do?_vl_backlink=/ home/ index. do) on 2011-02-10. . Retrieved 2010-08-22.
[140] "Ustawa z dnia 10 czerwca 2010 r. o zmianie ustawy o przeciwdziałaniu narkomanii [The Law of 10 June 2010 amending the Act on
Counteracting Drug Addiction]" (http:/ / isap. sejm. gov. pl/ DetailsServlet?id=WDU20101430962) (in Polish). Polish Internet Database
System of Legal Acts. 10 June 2010. . Retrieved 2010-08-25.
[141] Loh, L. (24 February 2010). "Legal in Singapore - the party drug that's banned everywhere else" (http:/ / www. cnngo. com/ singapore/
play/ mephedrone-party-drug-legal-singapore-535515). Cnngo.com. . Retrieved 2010-07-04.
[142] Yeo, A. (13 November 2010). "Three synthetic drugs to be banned" (http:/ / www. webcitation. org/ 5wOfQKDTu). Todayonline. Archived
from the original (http:/ / www. todayonline. com/ Singapore/ EDC101113-0000075/ Three-synthetic-drugs-to-be-banned) on 2011-02-10. .
Retrieved 2010-11-16.
[143] Hermsmeier, K. (28 August 2010). "Ich verfiel der Partydroge "Meow" [I fell in to the party drug "Meow"]" (http:/ / www. webcitation.
org/ 5wOffKgye). Archived from the original (http:/ / www. bz-berlin. de/ aktuell/ berlin/ ich-verfiel-der-partydroge-meow-article961726.
html) on 2011-02-10. . Retrieved 2010-09-21.
[144] Impey, J. (3 December 2010). "EU bans 'meow meow' party drug" (http:/ / www. webcitation. org/ 5wOjQQp9I). Deutsche Welle.
Archived from the original (http:/ / www. dw-world. de/ dw/ article/ 0,,6293967,00. html) on 2011-02-10. . Retrieved 2010-12-19.
[145] "Eldőlt: betiltják a katit [Decided: cathinones banned]" (http:/ / index. hu/ belfold/ 2010/ 09/ 02/ eldolt_betiltjak_a_katit/ ) (in Hungarian). 2
September 2010. . Retrieved 2011-01-02.
[146] "Magyar közlöny 2010. december 15. Kormányrendelet a Mephedrone listáravételéről. [Hungarian Government bans Mephedrone]" (http:/
/ www. kozlonyok. hu/ nkonline/ MKPDF/ hiteles/ MK10190. pdf) (in Hungarian). 15 December 2010. . Retrieved 2011-01-24.
[147] "'Miaow' drug seized in mail busts" (http:/ / www. webcitation. org/ 5wOfsndQN). Sydney Morning Herald. 12 February 2010. Archived
from the original (http:/ / www. smh. com. au/ national/ miaow-drug-seized-in-mail-busts-20100212-nwad. html) on 2011-02-10. . Retrieved
2010-09-21.
[148] Levy, M. (22 January 2011). "Judge puts users of new drug on notice" (http:/ / www. theage. com. au/ victoria/
judge-puts-users-of-new-drug-on-notice-20110121-1a02k. html). The Age. . Retrieved 2011-02-17.
[149] "Misuse of Drugs Act 1975 No 116, Public Act – New Zealand Legislation" (http:/ / www. webcitation. org/ 5wOip2edb). New Zealand
Ministry of Health. 1 December 2009. Archived from the original (http:/ / www. legislation. govt. nz/ act/ public/ 1975/ 0116/ latest/ whole.
html) on 2011-02-10. . Retrieved 2010-07-04.
[150] Edens, J. (14 September 2010). "Low-quality drugs prompt risk warning" (http:/ / www. webcitation. org/ 5wOiqFBfD). The Southland
Times. Archived from the original (http:/ / www. stuff. co. nz/ national/ health/ 4125151/ Low-quality-drugs-prompt-risk-warning) on
2011-02-10. . Retrieved 2010-09-21.
[151] "Controlled Drugs and Substances Act (1996, c. 19)" (http:/ / laws. justice. gc. ca/ eng/ C-38. 8/ FullText. html). Canadian Department of
Justice. 20 June 1996. . Retrieved 2011-01-29.
[152] Chan, Kevin (18 March 2010). "Mephedrone: From plant food to Britain’s party drug" (http:/ / www. webcitation. org/ 5wOfl6oz6). Globe
and Mail. Archived from the original (http:/ / www. theglobeandmail. com/ news/ world/ mephedrone-from-plant-food-to-britains-party-drug/
article1504199/ ) on 2011-02-10. . Retrieved 2010-03-23.
[153] Elwell, A. (26 April 2010). "Britain moves to curtail new drug craze" (http:/ / www. cmaj. ca/ cgi/ rapidpdf/ cmaj. 109-3245v1. pdf).
Canadian Medical Association Journal 182 (9): E393. doi:10.1503/cmaj.109-3245. PMC 2882479. PMID 20421358. .
[154] "U.K. party drug hits St. John's streets" (http:/ / www. cbc. ca/ canada/ newfoundland-labrador/ story/ 2010/ 06/ 25/
newfoundland-police-make-mephedrone-bust-625. html). CBC News. 25 June 2010. . Retrieved 2010-08-22.
[155] McCallum, J. (18 August 2010). "'Legal ecstasy' causing concern in Quebec" (http:/ / www. montrealgazette. com/ Legal+ ecstasy+
causing+ concern+ Quebec/ 3415148/ story. html). The Montreal Gazette. . Retrieved 2010-08-22.
[156] "RNC seizes designer drug" (http:/ / www. thetelegram. com/ Justice/ 2010-06-25/ article-1448526/ RNC-seizes-designer-drug/ 1). The
Telegram. 25 June 2010. . Retrieved 2010-08-22.
[157] "United States Drug Enforcement Agency Drug Scheduling" (http:/ / www. webcitation. org/ 5wOfpIRcB). Justice.gov. Archived from the
original (http:/ / www. justice. gov/ dea/ pubs/ scheduling. html) on 2011-02-10. . Retrieved 2010-07-04.
[158] "Drugs and Chemicals of Concern – 4-Methylmethcathinone" (http:/ / www. webcitation. org/ 5w9CmuHQx). United States Drug
Enforcement Agency. July 2010. Archived from the original (http:/ / www. deadiversion. usdoj. gov/ drugs_concern/ mephedrone. htm) on
2011-01-31. . Retrieved 2010-09-21.
[159] Sewell, A. (28 January 2011). "'Bath salts' latest drug to raise alarms" (http:/ / www. latimes. com/ news/ nationworld/ nation/
la-na-bath-salts-20110128,0,6453748,full. story). Los Angeles Times. . Retrieved 2011-02-17.
62
Mephedrone
External links
• Erowid 4-Methylmethcathinone Vault (http://www.erowid.org/chemicals/4_methylmethcathinone/)
• Mephedrone - Frequently asked questions (http://www.lifeline.org.uk/docs/Meph faq.pdf)
www.lifeline.org.uk
• Guardian Daily Podcast: How dangerous is mephedrone? (http://www.guardian.co.uk/world/audio/2010/
mar/19/guardian-daily-podcast-mephedrone-drug-meow)
• Mephedrone: A Musical Movement Or The Latest Fad? (http://www.rivmixx.com/interview/
feature-mephedrone/11619)
Norepinephrine releasing agent
A norepinephrine releasing agent (NRA) is a type of drug which induces the release of norepinephrine and
epinephrine from the pre-synaptic neuron into the synapse. This in turn leads to increased extracellular
concentrations of norepinephrine and and epinephrine therefore an increase in adrenergic neurotransmission.
Uses
NRAs are used for a variety of clinical indications including the following:
• For the treatment of attention-deficit hyperactivity disorder (ADHD) - e.g., amphetamine, methamphetamine,
pemoline.
• As anorectics in the treatment of obesity - e.g., amphetamine, phentermine, benzphetamine, phenmetrazine,
aminorex.
• As wakefulness-promoting agents in the treatment of narcolepsy - e.g., amphetamine, methamphetamine.
• As nasal decongestants - e.g., levomethamphetamine, propylhexedrine, ephedrine, pseudoephedrine,
phenylpropanolamine.
They are also used as recreational drugs, though this is typically reserved only for those that are also induce the
release of serotonin and/or dopamine like amphetamine, methamphetamine, MDMA, mephedrone, and
4-methylaminorex, among others.
Cathine and cathinone are NRAs found naturally in Catha edulis. Ephedrine and pseudoephedrine are also found
naturally in Ephedra sinica. Both of these plants are used medicinally (and recreationally as well regarding the
former). The endogenous trace amines phenethylamine and tyramine are NRAs found in many animals, including
humans.
References
63
Norepinephrine reuptake inhibitor
64
Norepinephrine reuptake inhibitor
A norepinephrine reuptake inhibitor (NRI, NERI) or adrenergic
reuptake inhibitor (ARI), is a type of drug which acts as a reuptake
inhibitor for the neurotransmitters norepinephrine (noradrenaline) and
epinephrine (adrenaline) by blocking the action of the norepinephrine
transporter (NET). This in turn leads to increased extracellular
concentrations of norepinephrine and epinephrine and therefore an
increase in adrenergic neurotransmission.
Indications
Epinephrine
NRIs may be used in the clinical treatment of attention-deficit hyperactivity disorder (ADHD), narcolepsy, and
fatigue or lethargy as stimulants, obesity as anorectics or appetite suppressants for weight loss purposes, as well as
mood disorders such as major depressive disorder (MDD) as antidepressants, nasal or sinus congestion as
decongestants, nocturnal enuresis or "bedwetting", hypotension and/or orthostatic hypotension as vasopressors, and
both as augmentations and to offset some of the side effects of certain other drugs like the selective serotonin
reuptake inhibitors (SSRIs) such as sexual dysfunction.
Effects
General
NRIs can induce a wide range of psychological and physiological effects, including the following:
Psychological
•
•
•
•
•
•
•
•
•
•
•
•
•
A general and subjective alteration in consciousness
Stimulation, arousal, and hyperactivity
Increased alertness, awareness, and wakefulness
Increased energy and endurance
Agitation or restlessness
Enhanced attention, focus, and concentration
Increased desire, drive, and motivation
Improved cognition, memory, and learning
Antidepressant benefits or mood lift
Irritability, aggression, anger and/or rage
Anxiety, negativity, paranoia, and/or panic attacks
Malaise or lassitude
Aphrodisiac effects
Physiological
•
•
•
•
•
Dizziness, lightheadedness, or vertigo
Mydriasis or pupil dilation
Xerostomia or dry mouth
Nasal and/or sinus decongestion
Nausea and/or emesis or vomiting
• Gastrointestinal disturbances
• Headache or migraine
Norepinephrine reuptake inhibitor
•
•
•
•
•
•
•
•
•
Trembling, shakiness, or muscle tremors
Diuretic effects or increased or frequent urination
Anorexia or decreased appetite and subsequent weight loss
Insomnia or inability to fall asleep
Analgesia or pain relief
Hypertension or increased blood pressure
Tachycardia or increased heart rate
Hyperthermia or increased body temperature
Hyperhidrosis or increased perspiration or sweating
Miscellaneous
• Drug tolerance with time and/or chronic administration, potentially resulting in dependence
• Drug interactions such as abolished effects from norepinephrine releasing agents like ephedrine
It should be noted, however, that many of these properties are dependent on whether the NRI in question is capable
of crossing the blood-brain-barrier (BBB). Those that do not will only produce peripheral effects.
Overdose
At very high doses characterized by overdose, a number of symptoms may come to prominence, as well as
hypertensive crisis, including the following:
Psychological
• Disorientation and/or confusion
• Severe anxiety, paranoia, and/or panic attacks
• Hypervigilance or increased sensitivity to perceptual stimuli, accompanied by significantly increased threat
detection
Physiological
•
•
•
•
•
•
•
•
•
•
•
Myoclonus or involuntary and intense muscle twitching
Hyperreflexia or overresponsive or overreactive reflexes
Tachypnoea or rapid breathing and/or dyspnea or shortness of breath
Palpitations or abnormal awareness of the beating of the heart
Angina pectoris or severe chest pain
Cardiac arrhythmia or abnormal electical activity of the heart
Circulatory shock or cardiogenic shock
Vasculitis or destruction of blood vessels
Cardiotoxicity or damage to the heart
Cardiac arrest, myocardial infarction or heart attack, and/or heart failure
Hemorrhage and/or stroke
Miscellaneous
•
•
•
•
Syncope or fainting or loss of consciousness
Seizures or convulsions
Neurotoxicity or brain damage
Coma and/or death
65
Norepinephrine reuptake inhibitor
Abuse
In contrast to dopamine reuptake inhibitors (DRIs) such as cocaine and methylphenidate, NRIs without DRI
properties which do not affect dopamine are incapable of inducing significant rewarding effects and are not
self-administered in rodents, and as a result, they have a negligible abuse potential in comparison.[1] [2]
List of NRIs
Pharmaceutical Drugs
• Selective Norepinephrine Reuptake Inhibitors (NRIs)
•
•
•
•
Atomoxetine/Tomoxetine (Strattera)
Mazindol (Mazanor, Sanorex)
Reboxetine (Edronax, Vestra)
Viloxazine (Vivalan)
• Norepinephrine-Dopamine Reuptake Inhibitors (NDRIs)
• Amineptine (Survector, Maneon, Directin)
• Bupropion (Wellbutrin, Zyban)
• Dexmethylphenidate (Focalin)
•
•
•
•
•
•
•
•
Fencamfamine (Glucoenergan, Reactivan)
Fencamine (Altimina, Sicoclor)
Lefetamine (Santenol)
Methylphenidate (Ritalin, Concerta, Metadate, Methylin)
Pipradrol (Meretran)
Prolintane (Promotil, Katovit)
Pyrovalerone (Centroton, Thymergix)
Difemetorex
• Serotonin-Norepinephrine Reuptake Inhibitors (SNRIs)
•
•
•
•
Desvenlafaxine (Pristiq)
Duloxetine (Cymbalta)
Milnacipran (Ixel, Savella)
Venlafaxine (Effexor)
• Tricyclic Antidepressants (TCAs)
•
•
•
•
•
•
•
•
•
•
•
Amitriptyline (Elavil)
Butriptyline (Evadyne)
Clomipramine (Anafranil)
Desipramine (Norpramin, Pertofrane)
Dosulepin (Prothiade)
Doxepin (Adapin, Sinequan)
Imipramine (Tofranil)
Lofepramine (Lomont, Gamanil)
Nortriptyline (Pamelor, Aventyl)
Protriptyline (Vivactil)
Trimipramine (Surmontil)
• Tetracyclic Antidepressants (TeCAs)
• Amoxapine (Asendin)
• Maprotiline (Ludiomil)
66
Norepinephrine reuptake inhibitor
67
• Mianserin (Bolvidon, Norval, Tolvon)
• Miscellaneous Agents
•
•
•
•
•
•
•
•
Cyclobenzaprine (Flexeril)
Mesocarb (Sidnocarb, Sydnocarb)
Nefazodone (Serzone) (weak)
Nefopam (Acupan)
Sibutramine (Meridia, Reductil)
Tapentadol (Nucynta)
Tramadol (Tramal, Ultram)
Ziprasidone (Geodon, Zeldox)
Dietary Supplements
• Adhyperforin (found in Hypericum perforatum (St. John's Wort))
• Hyperforin (found in Hypericum perforatum (St. John's Wort))
Street Drugs
• Cocaine (found in Erythroxylum coca (Coca))
• Desoxypipradrol (2-DPMP)
• Diphenylprolinol (D2PM)
• Methylenedioxypyrovalerone (MDPV)
Research Chemicals
• Selective Norepinephrine Reuptake Inhibitors (NRIs)
• Ciclazindol (Wy-23,409)
• Esreboxetine
• Manifaxine (GW-320,659)
• Nisoxetine (LY-94,939)
• Radafaxine (GW-353,162)
• Tandamine (AY-23,946)
• WYE-103231[3]
• [4]
• 1-(Indolin-1-yl)-1-phenyl-3-propan-2-olamines[5]
• 1- or 3-(3-Amino-2-hydroxy-1-phenyl
propyl)-1,3-dihydro-2H-benzimidazol-2-ones[6]
• (+)-S-21, thienyl-based[7]
• Serotonin-Norepinephrine-Dopamine Reuptake
Inhibitors (SNDRIs)
• Bicifadine (DOV-220,075)
•
•
•
•
•
•
•
•
•
Brasofensine (NS-2214)
Diclofensine (Ro-8-4650)
DOV-21,947
DOV-102,677
DOV-216,303
Indatraline (Lu-19-005)
NS-2359 (GSK-372,475)
Oxaprotiline (CGP-12,103-A)
SEP-225,289
Potent and selective NET ligands
Norepinephrine reuptake inhibitor
• SEP-227,162
• Tesofensine (NS-2330)
References
[1] Wee S, Woolverton WL (September 2004). "Evaluation of the reinforcing effects of atomoxetine in monkeys: comparison to methylphenidate
and desipramine". Drug and Alcohol Dependence 75 (3): 271–6. doi:10.1016/j.drugalcdep.2004.03.010. PMID 15283948.
[2] Gasior M, Bergman J, Kallman MJ, Paronis CA (April 2005). "Evaluation of the reinforcing effects of monoamine reuptake inhibitors under a
concurrent schedule of food and i.v. drug delivery in rhesus monkeys". Neuropsychopharmacology 30 (4): 758–64.
doi:10.1038/sj.npp.1300593. PMID 15526000.
[3] PMID 20462211
[4] PMID 20153188
[5] PMID 20131864
[6] PMID 19722525
[7] PMID 20378347
68
Propylhexedrine
69
Propylhexedrine
Propylhexedrine
Systematic (IUPAC) name
(RS)-N,α-dimethyl-cyclohexylethylamine
Identifiers
[1]
CAS number
3595-11-7
ATC code
??
PubChem
CID 7558
DrugBank
?
ChemSpider
7277
Synonyms
Hexahydrodesoxyephedrine; Hexahydromethamphetamine; Dimethylcyclohexaneethanamine
[2]
?
[3]
[4]
[5]
Chemical data
Formula
C H N
Mol. mass
155.29 g/mol
SMILES
eMolecules
10
21
[6]
& PubChem
[7]
Pharmacokinetic data
Bioavailability
?
Metabolism
?
Propylhexedrine
70
Half-life
?
Excretion
?
Therapeutic considerations
Pregnancy cat. C
Legal status
Unscheduled (US Federal)
Schedule varies (US States)
[8]
Schedule V (Canada Exempted from the application of the CDSA
Routes
Nasal inhaler, oral
(what is this?) (verify)
[9]
Propylhexedrine (Benzedrex, Dristan, Obesin) is a stimulant drug related to methamphetamine. It is used mainly
to provide temporary symptomatic relief of nasal congestion due to colds, allergies and allergic rhinitis. Being a
vasoconstrictor used to decongest nasal mucosa, it is administered by inhalation.
Propylhexedrine is most commonly found in over-the-counter Benzedrex and Dristan inhalers. Benzedrex was first
manufactured by Smith, Kline and French after the Benzedrine inhaler, which contained racemic amphetamine,
became unavailable following the placement of amphetamines on the U.S. Schedule II status (second highest abuse
potential with limited medicinal use). Propylhexedrine has also seen use in Europe as an appetite suppressant under
the trade name Obesin[10] and in the anticonvulsant preparation barbexaclone its S-isomer (levopropylhexedrine or
L-propylhexedrine) is bonded with phenobarbital for the purpose of offsetting the barbiturate-induced sedation.[10]
Levopropylhexedrine is also used as an anorectic under the brand name Eventin.
Medical use
Propylhexedrine is used for nasal congestion. Historically, it has also been used for weight loss.
Recreational use
Propylhexedrine has been reported to be used recreationally, obtained as freebase from the cotton rods that
Benzedrex inhalers contain.
Effects
The effects of propylhexedrine are similar to ephedrine or weak methamphetamine, although they usually vary from
person to person. Effects include increased sweating, talkativeness, euphoria, pupil dilation, emotional lability,
anorexia, tachycardia, palpitations, dry mouth, bruxism, anxiety, dysphoria, increased aggressiveness, paranoia,
headache, dizziness, slurred or impaired speech, rarely convulsions, serious heart problems; psychosis can occur.[11]
Withdrawal effects can occur and include fatigue, depression, suicidal tendencies, hunger and extreme desire for
sleep.
Recreational use potential
Propylhexedrine has a lower potential for abuse than other stimulants. This is partially due to the fact that methods of
its use are limited, unlike more commonly abused amphetamine, methamphetamine and methylphenidate. Oral
ingestion being the most practical method of consumption, the inactive ingredients in a Benzedrex inhaler (menthol
and lavender oil) are also ingested - most users report the taste and smell to be very unpleasant, resulting in "menthol
burps" (frequent belching releasing the smell of the two) that often cause the user discomfort and sometimes nausea.
Many drug users find that propylhexedrine has a very heavy "comedown" compared to the "high" it causes. The
abuse potential is considered to be low enough that neither the DEA nor the WHO consider it a drug of concern at
Propylhexedrine
the present, unlike ephedrine and its salts which are known to be used as precursor chemicals in illicit manufacture
of methamphetamine (and occasionally amphetamine). That said, propylhexedrine is controlled in some
jurisdictions.
Injection risks
While propylhexedrine is limited in a number of administration routes, attempts to extract the drug from the nasal
inhaler and then inject it have been reported. Recreational use by injection is dangerous and could result in serious
bodily harm or death. IV use of propylhexedrine is known to cause transient diplopia and brainstem dysfunction, and
deaths have been recorded in the medical literature. Typically, recorded cases of IV use are prepared by forming
propylhexedrine HCl in a solution with hydrochloric acid, the solution is then heated to evaporate and the resulting
crystals are dissolved in water for injection.[12] [13] [14]
Drug risks
As with similar drugs, using propylhexedrine to keep oneself awake for extended amounts of time can lead to a
temporary state of sleep deprivation during which an individual may experience hallucinations including auditory,
visual and tactile (e.g. bugs crawling on or under the skin), paranoia, irritability and impaired memory.
Propylhexedrine, being a vasocontrictor and a stimulant, may carry a further risk as blood pressure and heart rate are
raised, sometimes severely. Hypertension experienced by users of propylhexedrine can be dangerous, especially in
those who have pre-existing blood pressure problems. The increase in heart rate can lead to lower levels of oxygen,
discomfort, panic and in severe cases heart attack or serious arrhythmias. Again, this risk is higher if the user has
existing heart problems. Taking propylhexedrine and a MAOI together can lead to a fatal hypertensive crisis.
Propylhexedrine ingested along with other stimulants like amphetamines, caffeine and cocaine can lead to adverse
effects, such as serious hypertension and a significant increase in heart rate. It would be wise to avoid taking any
substance (including all recreational drugs, OTC and prescription medicines and herbal products) with
propylhexedrine, as interactions with are largely unknown.
Eating the cotton has its own risks, and while ingesting cotton is not necessarily fatal, it can lead to severe
gastrointestinal problems, which may require medical attention - cramps, gas and temporary constipation. More
serious problems can include gastric blockages and internal infection. Some users try to reduce this risk by shredding
the cotton into multiple pieces and swallowing at intervals; how effective this is in reducing the risk is unknown.
One way to avoid bowel blockages is to form an active salt of propylhexedrine and discard the cotton.
Chemistry
Propylhexedrine is structurally similar to methamphetamine. The only difference in the two compounds is that an
alicyclic cyclohexyl group is used in lieu of the aromatic phenyl group of methamphetamine. It is because of this that
propylhexedrine is not an amphetamine, nor even a phenethylamine, but instead can be referred to as a
cycloalkylamine.
Propylhexedrine, like amphetamine and methamphetamine, is a chiral compound (the α-carbon is chiral, like in its
amphetamine cousins). The propylhexedrine contained in Benzedrex inhalers is racemic (RS)-propylhexedrine as the
freebase. (S)-Propylhexedrine, also known as levopropylhexedrine, is believed to be the more biologically active
isomer of the two. [15] (S)-Propylhexedrine can be synthesized from dextro-methamphetamine.[16]
Freebase propylhexedrine is a volatile, oily liquid at room temperature. The slow vaporization of freebase
propylhexedrine allows it to be administered via inhalation.[17] Propylhexedrine hydrochloride is a white powder if
finely ground, or a clear crystalline substance if the crystals grown are larger.[18] The hydrochloride salt can be
vaporized much like the hydrochloride salt of methamphetamine can be.
71
Propylhexedrine
References
[1]
[2]
[3]
[4]
[5]
[6]
[7]
[8]
http:/ / www. nlm. nih. gov/ cgi/ mesh/ 2009/ MB_cgi?term=3595-11-7& rn=1
http:/ / www. whocc. no/ atc_ddd_index/ ?code=??
http:/ / pubchem. ncbi. nlm. nih. gov/ summary/ summary. cgi?cid=7558
http:/ / www. drugbank. ca/ drugs/ ?
http:/ / www. chemspider. com/ Chemical-Structure. 7277
http:/ / www. emolecules. com/ cgi-bin/ search?t=ex& q=N%28C%28CC1CCCCC1%29C%29C
http:/ / pubchem. ncbi. nlm. nih. gov/ search/ ?smarts=N%28C%28CC1CCCCC1%29C%29C
"Controlled Drugs and Substances Act" (http:/ / laws. justice. gc. ca/ en/ showdoc/ cs/ C-38. 8/ sc:5/ 20090817/ en#anchorsc:5). . Retrieved
20 August 2009.
[9] http:/ / en. wikipedia. org/ w/ index. php?& diff=cur& oldid=400868986
[10] Wesson DR (June 1986). "Propylhexdrine" (http:/ / linkinghub. elsevier. com/ retrieve/ pii/ 0376-8716(86)90013-X). Drug and Alcohol
Dependence 17 (2–3): 273–8. doi:10.1016/0376-8716(86)90013-X. PMID 2874970. .
[11] Fornazzari, L; Carlen, PL; Kapur, BM (1986). "Intravenous abuse of propylhexedrine (Benzedrex) and the risk of brainstem dysfunction in
young adults". The Canadian journal of neurological sciences. Le journal canadien des sciences neurologiques 13 (4): 337–9.
PMID 2877725.
[12] "Proposed Rules". Federal Register 50 (10): 2226–2227.
[13] Prince v. Ascher, 90 P.3d 1020 (2004).
[14] Fornazzari L, Carlen PL, Kapur BM. "Intravenous abuse of propylhexedrine (Benzedrex) and the risk of brainstem dysfunction in young
adults." Canadian Journal of Neurological Science. 1986 Nov;13(4):337-9. PMID 2877725
[15] A. M. Lands, V. L. Nash, H. R. Granger and B. L. Dertinger (1947). "The Pharmacologic Activity of N-Methyl-β-cyclohexylisopropylamine
Hydrochloride" (http:/ / jpet. aspetjournals. org/ content/ 89/ 3/ 382. short). JPET 89 (3): 382–385. .
[16] Textbook of organic medicinal and pharmaceutical chemistry (http:/ / books. google. com/ books?id=DczwAAAAMAAJ&
q=propylhexedrine+ prepared+ from+ methamphetamine& dq=propylhexedrine+ prepared+ from+ methamphetamine& hl=en&
ei=c9XhTM-zDYy-sAOCo-j2Cg& sa=X& oi=book_result& ct=result& resnum=3& ved=0CC0Q6AEwAg), Charles Owens Wilson, Ole
Gisvold, Robert F. Doerge, page 491
[17] Nasal Inhaler (http:/ / www. google. com/ patents?hl=en& lr=& vid=USPAT4095596& id=W3I1AAAAEBAJ& oi=fnd&
dq=propylhexedrine+ inhalation& printsec=abstract#v=onepage& q& f=false), US 4095596 (http:/ / v3. espacenet. com/
textdoc?DB=EPODOC& IDX=US4095596)
[18] Mancusi-Ungaro, H. R. Jr. M.D.; Decker, W. J. Ph.D.; Forshan, V. R. D. O.; Blackwell, S. J. M.D.; Lewis, S. R. M.D. (1983). "Tissue
Injuries Associated With Parenteral Propylhexedrine Abuse" (http:/ / journals. lww. com/ jtrauma/ Citation/ 1983/ 07000/
Tissue_Injuries_Associated_With_Parenteral. 114. aspx). Journal of Trauma-Injury Infection & Critical Care 23 (7): 650.
doi:10.1097/00005373-198307000-00114. .
72
Psychoanaleptic
73
Psychoanaleptic
In pharmacology, a psychoanaleptic is a medication which produces an arousing effect upon the patient.
Antidepressants, psychostimulants, agents used for ADHD and nootropics and Anti-dementia drugs are all
psychoanaleptics.
The psychoanaleptics are classified under N06 in the Anatomical Therapeutic Chemical Classification System.
External links
• http://medical-dictionary.thefreedictionary.com/psychoanaleptic
Selective serotonin releasing agent
A selective serotonin releasing agent (SSRA) is a type of drug which selectively induces the release of serotonin
from the pre-synaptic neuron into the synapse. This in turn leads to increased extracellular concentrations of
serotonin and therefore a selective increase in serotonergic neurotransmission.
SSRAs have been used as anorectics or appetite suppressants in the past, and they have also been suggested as novel
antidepressant and anxiolytic agents with a faster onset and superior efficacy in comparison to the selective serotonin
reuptake inhibitors (SSRIs).[1]
The term 'selective serotonin releasing agent' was coined by David E. Nichols.[2]
List of SSRAs
Pharmaceutical Drugs
• Chlorphentermine (Apsedon, Desopimon, Lucofen)
• Cloforex (Oberex; prodrug to chlorphentermine)
• Dexfenfluramine (Redux)
• Etolorex (prodrug to chlorphentermine; never marketed)
• Fenfluramine (Pondimin, Fen-Phen)
• Norfenfluramine (metabolite of fenfluramine; never marketed)
Research Chemicals
• 2-Methyl-3,4-methylenedioxyamphetamine (2-Methyl-MDA)
• 3-Methoxy-4-methylamphetamine (MMA)
• 3-Methyl-4,5-methylenedioxyamphetamine (5-Methyl-MDA)
• 3,4-Ethylenedioxy-N-methylamphetamine (EDMA)
• 4-Methoxyamphetamine (PMA)
•
•
•
•
•
•
•
4-Methoxy-N-ethylamphetamine (PMEA)
4-Methoxy-N-methylamphetamine (PMMA)
4-Methylthioamphetamine (4-MTA)
5-(2-Aminopropyl)-2,3-dihydrobenzofuran (5-APDB)
5-Indanyl-2-aminopropane (IAP)
5-Methoxy-6-methylaminoindane (MMAI)
5-Trifluoromethyl-2-aminoindane (TAI)
• 5,6-Methylenedioxy-2-aminoindane (MDAI)
• 5,6-Methylenedioxy-N-methyl-2-aminoindane (MDMAI)
Fenfluramine, the prototypical SSRA,
previously used as an anorectic before being
withdrawn from the market due to toxicity
concerns.
Selective serotonin releasing agent
•
•
•
•
•
•
74
6-Chloro-2-aminotetralin (6-CAT)
6-Tetralinyl-2-aminopropane (TAP)
6,7-Methylenedioxy-2-aminotetralin (MDAT)
6,7-Methylenedioxy-N-methyl-2-aminotetralin (MDMAT)
N-Ethyl-5-trifluoromethyl-2-aminoindane (ETAI)
N-Methyl-5-indanyl-2-aminopropane (IMP)
References
[1] Scorza C, Silveira R, Nichols DE, Reyes-Parada M (July 1999). "Effects of 5-HT-releasing agents on the extracellullar hippocampal 5-HT of
rats. Implications for the development of novel antidepressants with a short onset of action" (http:/ / linkinghub. elsevier. com/ retrieve/ pii/
S0028390899000234). Neuropharmacology 38 (7): 1055–61. doi:10.1016/S0028-3908(99)00023-4. PMID 10428424. .
[2] Marona-Lewicka D, Nichols DE (June 1994). "Behavioral effects of the highly selective serotonin releasing agent
5-methoxy-6-methyl-2-aminoindan" (http:/ / linkinghub. elsevier. com/ retrieve/ pii/ 0014-2999(94)90051-5). European Journal of
Pharmacology 258 (1-2): 1–13. doi:10.1016/0014-2999(94)90051-5. PMID 7925587. .
Serotonin releasing agent
A serotonin releasing agent (SRA) is a type of drug which
induces the release of serotonin from the pre-synaptic neuron into
the synapse. This in turn leads to increased extracellular
concentrations of serotonin and therefore an increase in
serotonergic neurotransmission.
Clinical and recreational use
MDMA, MDEA, MDA, and MBDB, among other relatives (see
MDxx), are recreational drugs termed empathogen-entactogens
and are often used at all-night dance parties known as raves. They
act as releasing agents of not only serotonin, but of dopamine and
norepinephrine as well.
Serotonin
Fenfluramine, chlorphentermine, and aminorex were used as appetite suppressants but they were discontinued due to
concerns of cardiac valvulopathy. This side effect has been attributed not only to their action as SRAs but due to
potent agonism of the 5-HT2B receptor as well. The designer drugs MDMA and 4-methylaminorex which are also
SRAs and 5-HT2B agonists have been reported to cause this effect as well.
Tramadol, in addition to its opioid and norepinephrine reuptake-inhibiting effects, is an SRA and is used as an
analgesic. Indeloxazine is an SRA and norepinephrine reuptake inhibitor used as a nootropic and neuroprotective.
αET and αMT are serotonin, norepinephrine, and dopamine releasing agents which were formerly used as
antidepressants in Russia. They are now encountered solely as recreational drugs similarly to the MDxx series.
Serotonin releasing agent
List of SRAs
Pharmaceutical Drugs
• Amphetamines
• Chlorphentermine (Apsedon, Desopimon, Lucofen)
• Cloforex (Oberex)
• Clortermine (Voranil)
• Dexfenfluramine (Redux)
• Fenfluramine (Pondimin, Fen-Phen)
• Norfenfluramine (metabolite of fenfluramine)
• Oxazolines
• Aminorex (Menocil)
• Tryptamines
• Etryptamine (Monase)[1]
• Metryptamine (Indopan)[2] [2] [3]
• Others
• Indeloxazine (Elen, Noin)[4]
• Tramadol (Ultram, Tramal)[5] [6] [7]
Street Drugs
• Amphetamines
• 4-Methylthioamphetamine (4-MTA)
• Methylenedioxyamphetamine (MDA)
• Methylenedioxyethylamphetamine (MDEA)
• Methylenedioxymethamphetamine (MDMA; "Ecstasy")[2]
• para-Methoxyamphetamine (PMA)
• para-Methoxyethylamphetamine (PMEA)
• para-Methoxymethamphetamine (PMMA)
• Cathinones
• Butylone
• Ethylone
• Mephedrone
• Methedrone
• Methylone[2]
• Oxazolines
• 4-Methylaminorex (4-MAR)
• Piperazines
• meta-Chlorophenylpiperazine (mCPP)[2] [8] [9]
• Methoxyphenylpiperazine (MeOPP)[2]
• para-Fluorophenylpiperazine (pFPP; Fluoperazine)
• Trifluoromethylphenylpiperazine (TFMPP)
• Tryptamines
• 5-Methoxyalphaethyltryptamine (5-MeO-AET)
• 5-Methoxyalphamethyltryptamine (5-MeO-AMT)[2]
Research Chemicals
• Aminoindanes
75
Serotonin releasing agent
• 5-Iodoaminoindane (5-IAI)
• Ethyltrifluoromethylaminoindane (ETAI)
• Indanylaminopropane (IAP)
• Indanylmethylaminopropane (IMP)
• Methoxymethylaminoindane (MMAI)
• Methylenedioxyaminoindane (MDAI)
• Methylenedioxymethylaminoindane (MDMAI)
• Trifluoromethylaminoindane (TAI)
• Aminotetralins
• 8-Hydroxydipropylaminotetralin (8-OH-DPAT)[10]
• 6-Chloroaminotetralin (6-CAT)
• Methylenedioxyaminotetralin (MDAT)
• Amphetamines
•
•
•
•
3-Methylamphetamine (3-MA)
4-Methylamphetamine (4-MA)
4-Methylmethamphetamine (4-MMA)
5-(2-Aminopropyl)-2,3-dihydrobenzofuran (5-APDB)
• 6-(2-Aminopropyl)-2,3-dihydrobenzofuran (6-APDB)
• Amiflamine
• Benzodioxylbutanamine (BDB)[2]
• Ethylenedioxymethamphetamine (EDMA)
• Etolorex
• Methoxymethylamphetamine (MMA)
• Methoxymethylenedioxyamphetamine (MMDA)
• Methoxymethylenedioxymethylamphetamine (MMDMA)
• Methylbenzodioxolbutanamine (MBDB)[2]
• Methylenedioxyhydroxyamphetamine (MDOH)
• Methylenedioxyhydroxymethamphetamine (MDHMA)
• para-Bromoamphetamine (PBA)
• para-Chloroamphetamine (PCA)
• para-Iodoamphetamine (PIA)
• Tryptamines
• 5-Carboxamidotryptamine (5-CT)[10]
• 5-Methoxytryptamine (5-MT)[10]
• Others
• Naphthylaminopropane (NAP; PAL-287)
Trace Amines
• Tryptamine[10]
76
Serotonin releasing agent
References
[1] Krebs KM, Geyer MA (1993). "Behavioral characterization of alpha-ethyltryptamine, a tryptamine derivative with MDMA-like properties in
rats". Psychopharmacology 113 (2): 284–7. doi:10.1007/BF02245712. PMID 7855195.
[2] Nagai F, Nonaka R, Satoh Hisashi Kamimura K (March 2007). "The effects of non-medically used psychoactive drugs on monoamine
neurotransmission in rat brain". European Journal of Pharmacology 559 (2-3): 132–7. doi:10.1016/j.ejphar.2006.11.075. PMID 17223101.
[3] Marsden CA (November 1979). "Evidence for the release of hippocampal 5-hydroxytryptamine by alpha-methyltryptamine [proceedings]".
British Journal of Pharmacology 67 (3): 438P–439P. PMC 2043998. PMID 497560.
[4] Yamaguchi T, Ohyama M, Suzuki M, et al. (September 1998). "Neurochemical and behavioral characterization of potential antidepressant
properties of indeloxazine hydrochloride" (http:/ / linkinghub. elsevier. com/ retrieve/ pii/ S0028390898000094). Neuropharmacology 37 (9):
1169–76. doi:10.1016/S0028-3908(98)00009-4. PMID 9833647. .
[5] Driessen B, Reimann W (January 1992). "Interaction of the central analgesic, tramadol, with the uptake and release of 5-hydroxytryptamine
in the rat brain in vitro". British Journal of Pharmacology 105 (1): 147–51. PMC 1908625. PMID 1596676.
[6] Bamigbade TA, Davidson C, Langford RM, Stamford JA (September 1997). "Actions of tramadol, its enantiomers and principal metabolite,
O-desmethyltramadol, on serotonin (5-HT) efflux and uptake in the rat dorsal raphe nucleus" (http:/ / bja. oxfordjournals. org/ cgi/
pmidlookup?view=long& pmid=9389855). British Journal of Anaesthesia 79 (3): 352–6. PMID 9389855. .
[7] Reimann W, Schneider F (May 1998). "Induction of 5-hydroxytryptamine release by tramadol, fenfluramine and reserpine" (http:/ /
linkinghub. elsevier. com/ retrieve/ pii/ S0014-2999(98)00195-2). European Journal of Pharmacology 349 (2-3): 199–203.
doi:10.1016/S0014-2999(98)00195-2. PMID 9671098. .
[8] Eriksson E, Engberg G, Bing O, Nissbrandt H (March 1999). "Effects of mCPP on the extracellular concentrations of serotonin and dopamine
in rat brain". Neuropsychopharmacology : Official Publication of the American College of Neuropsychopharmacology 20 (3): 287–96.
doi:10.1016/S0893-133X(98)00070-0. PMID 10063489.
[9] Baumann MH, Ayestas MA, Dersch CM, Rothman RB (May 2001). "1-(m-chlorophenyl)piperazine (mCPP) dissociates in vivo serotonin
release from long-term serotonin depletion in rat brain". Neuropsychopharmacology : Official Publication of the American College of
Neuropsychopharmacology 24 (5): 492–501. doi:10.1016/S0893-133X(00)00221-9. PMID 11282249.
[10] Wölfel R, Graefe KH (February 1992). "Evidence for various tryptamines and related compounds acting as substrates of the platelet
5-hydroxytryptamine transporter". Naunyn-Schmiedeberg's Archives of Pharmacology 345 (2): 129–36. doi:10.1007/BF00165727.
PMID 1570019.
Further reading
• Nichols DE, Marona-Lewicka D, Huang X, Johnson MP (1993). "Novel serotonergic agents". Drug Design and
Discovery 9 (3-4): 299–312. PMID 8400010.
• Marona-Lewicka D, Nichols DE (December 1997). "The Effect of Selective Serotonin Releasing Agents in the
Chronic Mild Stress Model of Depression in Rats". Stress (Amsterdam, Netherlands) 2 (2): 91–100.
doi:10.3109/10253899709014740. PMID 9787258.
• Scorza C, Silveira R, Nichols DE, Reyes-Parada M (July 1999). "Effects of 5-HT-releasing agents on the
extracellullar hippocampal 5-HT of rats. Implications for the development of novel antidepressants with a short
onset of action" (http://linkinghub.elsevier.com/retrieve/pii/S0028390899000234). Neuropharmacology 38
(7): 1055–61. doi:10.1016/S0028-3908(99)00023-4. PMID 10428424.
• Rothman RB, Baumann MH (April 2002). "Serotonin releasing agents. Neurochemical, therapeutic and adverse
effects" (http://linkinghub.elsevier.com/retrieve/pii/S0091305701006694). Pharmacology, Biochemistry, and
Behavior 71 (4): 825–36. doi:10.1016/S0091-3057(01)00669-4. PMID 11888573.
77
Serotonin reuptake inhibitor
Serotonin reuptake inhibitor
A serotonin reuptake inhibitor (SRI) is a type of drug which
acts as a reuptake inhibitor for the neurotransmitter serotonin
(5-hydroxytryptamine (5-HT)) by blocking the action of the
serotonin transporter (SERT). This in turn leads to increased
extracellular concentrations of serotonin and therefore an increase
in serotonergic neurotransmission.
SRIs are not synonymous with selective serotonin reuptake
inhibitors (SSRIs), as the latter term is usually used to describe the
class of antidepressants of the same name, and, because SRIs,
Serotonin
unlike SSRIs, can be either selective or nonselective in their
action. For example, cocaine, which nonselectively inhibits the
reuptake of serotonin, norepinephrine, and dopamine, can obviously be called an SRI, but not an SSRI.
Indications
SRIs may be used in the clinical treatment of mood disorders such as major depressive disorder (MDD), dysthymia,
and bipolar disorder (BD) as antidepressants, anxiety disorders such as generalized anxiety disorder (GAD), social
phobia (SP) also known as social anxiety disorder (SAD), obsessive-compulsive disorder (OCD), and panic disorder
(PD) as anxiolytics, post-traumatic stress disorder (PTSD), body dysmorphic disorder (BDD), eating disorders like
anorexia nervosa and bulimia nervosa, and certain personality disorders such as borderline personality disorder
(BPD), as well as chronic pain, neuralgia or neuropathic pain, and fibromyalgia as analgesics (see duloxetine,
milnacipran, and bicifadine), irritable bowel syndrome (IBS) as gastroprokinetic agents, premature ejaculation (PE)
(see dapoxetine), drug addiction as anticraving agents, and obesity for anorectic or weight loss purposes (see
sibutramine).
Effects
General
SRIs can induce a wide range of psychological and physiological effects, including the following:
Psychological
•
•
•
•
•
•
•
•
•
•
•
A general and subjective alteration in consciousness
Sedation or drowsiness
Somnolence or sleepiness
Fatigue or lethargy
Malaise or lassitude
Agitation or restlessness
Akathisia or unpleasant sensations of "inner restlessness" and inability to sit still or remain motionless
Decreased alertness, awareness, and wakefulness
Impaired attention, focus, and concentration
Decreased drive and motivation
Cognitive and memory impairment
• Antidepressant benefits or mood lift
• Anxiolysis and/or stress reduction
• Apathy and/or anhedonia or emotional blunting
78
Serotonin reuptake inhibitor
•
•
•
•
•
Euphoria and/or dysphoria
Antiaggressive or serenic effects, or even entactogen qualities
Vivid, bizarre, or strange dreams, or a suppression of dreaming
Psychedelia, consisting of mental imagery, auditory and visual hallucinations and distortions, and synesthesia
Paradoxical exacerbation of depression and/or anxiety, as well as suicidal ideation, though usually only acute in
nature
Physiological
•
•
•
•
•
•
•
•
•
•
Dizziness, lightheadedness, or vertigo
Blurry vision and/or nystagmus or involuntary and rapid eye movements
Mydriasis or pupil dilation
Xerostomia or dry mouth
Nausea and/or emesis or vomiting
Gastrointestinal disturbances such as diarrhea and/or constipation
Headache or migraine
Photosensitivity or increased risk of sunburn
Trismus or jaw clenching and/or bruxism or teeth grinding
Trembling, shakiness, or muscle temors
• Restless legs syndrome (RLS)
• Antidiuretic effects and/or ischuria or urinary retention or difficulting urinating
• Sexual dysfunction, consisting of diminished libido, soft erections or erectile dysfunction, and anorgasmia or
inability to ejaculate
• Anorexia or decreased appetite and subsequent weight loss
• Insomnia or inability to fall asleep
• Analgesia or pain relief
• Hypertension or increased blood pressure
• Tachycardia or increased heart rate
• Hyperthermia or increased body temperature
• Hyperhidrosis or increased perspiration or sweating
• Autonomic dysfunction such as orthostatic hypotension
• Hyponatremia or a water-electrolyte imbalance
• Liver or kidney dysfunction, toxicity, or failure
Miscellaneous
• Decreased drug cravings and/or addiction
• Drug tolerance with time and/or chronic administration, potentially resulting in dependence
• Drug interactions such as abolished effects from serotonin releasing agents (SRAs) like MDMA ("Ecstasy")
It should be noted, however, that many of these effects are dependent on whether the administration of the SRI is
acute or chronic. As an example, acute ingestion typically does not result in a mood lift, and chronic administration
usually does not result in any sort of psychedelia whatsoever. Additionally, many of these properties are also
dependent on whether the SRI in question is capable of crossing the blood-brain-barrier (BBB). Those that do not
will only produce peripheral effects.
79
Serotonin reuptake inhibitor
Overdose
At very high doses characterized by overdose, serotonin syndrome may develop, resulting in symptoms including the
following:
Psychological
• Disorientation and/or confusion
• Anxiety, paranoia, and/or panic attacks
• Hypervigilance or increased sensitivity to perceptual stimuli, accompanied by significantly increased threat
detection
• Hypomania or full-blown mania
• Derealization and/or depersonalization
• Hallucinations and/or perceptual disturbances
Physiological
•
•
•
•
Myoclonus or involuntary and intense muscle twitching
Hyperreflexia or overresponsive or overreactive reflexes
Tachypnoea or rapid breathing
Chest pain and/or pulmonary hypertension (PH)
• Severe hyperthermia, potentially resulting in organ failure
Miscellaneous
•
•
•
•
•
Syncope or fainting or loss of consciousness
Seizures or convulsions
Organ failure (as mentioned above)
Neurotoxicity or brain damage
Coma and/or death
List of SRIs
Pharmaceutical Drugs
• Selective Serotonin Reuptake Inhibitors (SSRIs)
•
•
•
•
•
•
•
•
•
•
Citalopram (Celexa)
Dapoxetine (Priligy)
Escitalopram (Lexapro, Cipralex)
Femoxetine (Malexil)
Fluoxetine (Prozac)
Fluvoxamine (Luvox)
Indalpine (Upstene)
Paroxetine (Paxil, Seroxat)
Sertraline (Zoloft, Lustral)
Zimelidine (Normud, Zelmid)
• Serotonin-Norepinephrine Reuptake Inhibitors (SNRIs)
•
•
•
•
Desvenlafaxine (Pristiq)
Duloxetine (Cymbalta)
Milnacipran (Ixel, Savella)
Venlafaxine (Effexor)
• Tricyclic Antidepressants (TCAs)
• Amitriptyline (Elavil)
80
Serotonin reuptake inhibitor
•
•
•
•
•
•
•
•
•
•
Butriptyline (Evadyne)
Clomipramine (Anafranil)
Dibenzepin (Noveril)
Dosulepin (Prothiade)
Doxepin (Adapin, Sinequan)
Imipramine (Tofranil)
Lofepramine (Lomont, Gamanil)
Nortriptyline (Pamelor, Aventyl)
Protriptyline (Vivactil)
Trimipramine (Surmontil)
• Tetracyclic Antidepressants (TeCAs)
• Amoxapine (Asendin)
• Opioid Analgesics
• Meperidine/Pethidine (Demerol)[1]
• Methadone (Dolophine, Methadose)[1]
• Propoxyphene (Darvon)[1]
• First-Generation Antihistamines
•
•
•
•
Chlorpheniramine (Chlor-Trimeton, etc.)[2]
Diphenhydramine (Benadryl, etc.)[2]
Mepyramine/Pyrilamine (Anthisan, etc.)[2]
Tripelennamine (Pyribenzamine, etc.)[2]
• Miscellaneous Agents
•
•
•
•
•
•
•
•
Cyclobenzaprine (Flexeril)
Dextromethorphan (DXM; Robitussin, etc.)[3]
Dextrorphanol (DXO) (an active metabolite of DXM)
Nefazodone (Serzone)
Nefopam (Acupan)
Sibutramine (Meridia, Reductil)
Trazodone (Desyrel)
Ziprasidone (Geodon, Zeldox)
Dietary Supplements
• Adhyperforin (found in Hypericum perforatum (St. John's Wort))
• Hyperforin (found in Hypericum perforatum (St. John's Wort))
• Mesembrine (found in Sceletium tortuosum (Kanna))[4]
Street Drugs
• Cocaine (found in Erythroxylum coca (Coca))
Research Chemicals
•
•
•
•
•
Alaproclate (GEA-654)
Bicifadine (DOV-220,075)
Brasofensine (NS-2214)
Bromantane (ADK-709)
Diclofensine (Ro-8-4650)
• DOV-21,947
• DOV-102,677
81
Serotonin reuptake inhibitor
•
•
•
•
•
•
•
•
•
•
•
•
DOV-216,303
Indatraline (Lu-19-005)
Litoxetine (SL-810,385)
Lubazodone (YM-992, YM-35,995)
NS-2359 (GSK-372,475)
SB-649,915
SEP-225,289
SEP-227,162
Tametraline (CP-24,411)
Tesofensine (NS-2330)
Vilazodone (EMD-68,843)
Viqualine (PK-5078)
References
[1] Gillman PK. (2005). "Monoamine oxidase inhibitors, opioid analgesics and serotonin toxicity.". Br J Anaesth. 95 (4): 434–41.
doi:10.1093/bja/aei210. PMID 16051647.
[2] Yeh SY, Dersch C, Rothman R, Cadet JL (September 1999). "Effects of antihistamines on 3, 4-methylenedioxymethamphetamine-induced
depletion of serotonin in rats". Synapse 33 (3): 207–17. doi:10.1002/(SICI)1098-2396(19990901)33:3<207::AID-SYN5>3.0.CO;2-8.
PMID 10420168.
[3] Werling LL, Keller A, Frank JG, Nuwayhid SJ (2007). "A comparison of the binding profiles of dextromethorphan, memantine, fluoxetine
and amitriptyline: treatment of involuntary emotional expression disorder". Exp Neurol. 207 (2): 248–57.
doi:10.1016/j.expneurol.2007.06.013. PMID 17689532.
[4] Pharmaceutical compositions containing mesembrine and related compounds. U.S. Patent 6,288,104 (http:/ / patft. uspto. gov/ netacgi/
nph-Parser?patentnumber=6,288,104)
82
Article Sources and Contributors
Article Sources and Contributors
Adenosine reuptake inhibitor Source: http://en.wikipedia.org/w/index.php?oldid=418195470 Contributors: El3ctr0nika, Greedyhalibut, Pustelnik, Yikrazuul
Amphetamine Source: http://en.wikipedia.org/w/index.php?oldid=430321995 Contributors: (jarbarf), *Kat*, 11bje11, 130.60.68.xxx, 2T, 2over0, 65.24.160.xxx, A.Arc, AThing, Aborell,
Abrech, Adamfinmo, Addicted2l, Addshore, Adouglass, Afasmit, Agnte, Airplaneman, Ajax151, Akamad, Akirasplace, Al Wiseman, Alansohn, Alchie1, Alfie66, Allethrin, Allstarecho,
Alphachimera, Anaraug, Anareon, Andonic, Andy M. Wang, Andycjp, Angel caboodle, Anil1956, AnnaFrance, Annanda, Antandrus, Anton42, Arcadian, ArdClose, Aristotle1990, Arjuna,
Arthena, Ascidian, Ashleyisachild, Astral, Astral Zen, Auranor, Avalyn, AxelBoldt, BBLove, BD2412, Bdesham, Bearingbreaker92, Beetstra, Belovedfreak, Benjah-bmm27, Benwards1,
Benzenerings, Bethwilk, Betohaku, Bezwenalife, Bitoffish, Black Platypus, Blanchardb, BlastOButter42, Blue bear sd, Blurpeace, Bob diablo, Bobblewik, Bobo192, Bogdangiusca, Bohemian
Arcade, BoomerAB, Borgx, Bped1985, Briskat, Bronckobuster, Bruce1ee, Bryan Derksen, Bustermythmonger, C6541, CMBJ, COMPFUNK2, CPS, CWii, Cacycle, Cadmium, Calvados,
CambridgeBayWeather, Cameron1194, Can't sleep, clown will eat me, CanisRufus, CarbonUnit, CardinalDan, Carmichael95, Casforty, Cdc, Cedders, Cerebrith, Cessator, Chal09, Chanting Fox,
CharlesC, CharlotteWebb, Chasingsol, CheeseNoodles, ChemNerd, Chesnok, Cheveux, Chezulvena, Chowbok, Chris 73, Chris Henniker, ChrisGriswold, Chriswiki, Cimarcia, Citysave, Clovis
Sangrail, Conversion script, Corvus cornix, CosineKitty, Cowpepper, Cpkoch, Cpl Syx, Craigsjones, Crakkpot, Crazytonyi, Cregq, CrookedAsterisk, Cst17, Cuvtixo, Cyde, DFerret, DMCer,
Damian Yerrick, DanMatan, Danielt, Danorton, David Sulzer, David0811, David51387, Davidlawrence, Dbtfz, Dcflyer, Ddhix 2002, DeadEyeArrow, Debollweevil, Debug2007, Dekisugi, Deli
nk, Delighted eyes, Delta G, Derek Ross, Descendall, Dextrodamien, Diberri, Dl2000, Dowcet, Dpspendragon, Dr bab, Dr.K., DriverDan, Drphilharmonic, DryGrain, Dryman, Dying2live,
Dylancatlow, Dynaflow, Earle Martin, Edgar181, Editor182, Edward Bower, Eequor, Eggman64, El3ctr0nika, ElBenevolente, Elangsto, ElectricValkyrie, Element16, Elias Zandi, Elmozo, Eloil,
Euanmacdermid, Eurocrat123, Evanreyes, Everything Else Is Taken, Ewlyahoocom, Extransit, Fabrício Kury, Father McKenzie, Fearisstrong, Fenke, Fieldday-sunday, Filcollins, FireFish,
Foiltape, Foolishben, Footballdec, Fsdadssghfd, Funandtrvl, Funkbrother3000, Furrykef, Fuzbaby, Fuzzform, Fvasconcellos, Fxcastellanos, Fyyer, Gabbe, Gabriel.Villeda, Gaelen S., Gaius
Cornelius, Galaxiaad, Gettingtoit, Gilliam, Gobonobo, Gogo Dodo, GraemeL, Gravitan, Green30, Guitarmankev1, Gurch, Gustavb, Gwernol, Gyan, Gyrofrog, Hagerman, Halmstad, Halo,
Haniel777, Harbinary, Harryboyles, Haza-w, HazyM, Heah, Heathniederee, Hede2000, Helgihg, Heymon32, Hgspring, Hiberniantears, Hindzyboy93, Hkazmi, Hli, Hoponpop69, Hoyiu,
Hughcharlesparker, IW.HG, Iainhammer, Icecreammaninthegarbagecan, Icseaturtles, IllusionalFate, Imroy, Insaneclown510, Insidious1, Intesvensk, Irishrichy, Irmgard, Islam123098, J.delanoy,
JForget, JMK, JWSchmidt, Ja 62, JaGa, Jamesters, Jamie wilkinson smith, Jatlas, Jeff G., Jeffq, JetLover, Jetekus, Jfurr1981, Jhaochen, JidGom, Jimebob, Jiy, Jkhamlin, Jmcclung711, Jmh649,
Jobo123, JoeSmack, Joejello, John Cho, John Vandenberg, JohnBlackburne, JohnCD, Jojhutton, JonM.D., Joshlepaknpsa, Joshlmay, Jpbrenna, Jugger90, Julesd, Jusdafax, Just Another Dan, Jü,
K10wnsta, Karl gregory jones, Kasey1123, Kassem23, Katieh5584, KayEss, Keilana, Keith H., Killergreenleg, Kiteinthewind, Knightfever17, KonradG, Kpjas, Krellis, Kslays, L Kensington,
Laurel Bush, Leadwind, Leahtwosaints, LeaveSleaves, Lectric1, Liam Skoda, Ligulem, Lilypopluiy, Liviak, Lmatt, Localzuk, Looxix, Louisajb, Luna Santin, MONGO, MagicOgre,
Magicandmyth, Magioladitis, Mangojuice, Marcoel, Marcus22, Mark K. Jensen, Mark PEA, Marqueed, Mary quite contrary, MattKingston, MaxErdwien, Maximus Rex, Maxtitan, Me86532,
Meco, Mediatiger838, Medos2, Meekywiki, MegX, Meodipt, Merovingian, Mert91, Mikael Häggström, MikeCU2008, MilkMiruku, Mirv, Miserlou, Mizukind, Mizushimo, Mkaufman,
Mmortal03, Monkeybubbles, Mr Bungle, Mr. Anon515, MttJocy, [email protected], Muneyama, Mushin, Nagelfar, Nappymonster, Natalie Erin, NattoMaki, NawlinWiki, Negaluke,
Neilc, Netscott, Nick Drake, Nick Number, Nihiltres, Ninaf09, Nirmos, Nishaddatta, Nishkid64, Nivix, Nixdorf, Nixeagle, Noodles32236, Nsaa, NuclearWarfare, Nutriveg, Nvcozzi, ONEder
Boy, ObiusX, Obuolys, Octavio L, Ohnoitsjamie, Ohnoitsthefuzz, OlEnglish, Oluicsaram2, Onthegogo, Oscarthecat, PCock, PStrait, Pacula, Panyd, ParisianBlade, Pashihiko, PasswordUsername,
Pavel Vozenilek, Pb30, Pdcook, Peak, Pearle, Pedro, Pen1234567, Pgk, Pharmacophore, Phil Boswell, Phoenix2, PiCo, PigFlu Oink, Piperh, Pjvpjv, Politepunk, Pontificalibus, Prodego,
PseudoSudo, Psherid1, Quadell, Quihn, Quinsareth, Quintote, Quirk, R'n'B, RDBrown, RFlynn26, RJFJR, Rada, RainbowOfLight, Rakkar, Ral315, Rbaselt, Rdsmith4, Red Director, Redfirefly,
Reedy, Refault, Remember me, RepublicanJacobite, Retired username, RevRagnarok, Riana, Rich Farmbrough, Richard L. Peterson, RickyTheJanitor, Ridethatpony35, Rifleman 82, Rintrah,
Rjwilmsi, Roadrunner, Roberta F., Rodeosmurf, Rogertudor, Roghro, Rookkey, RoyBoy, Roybristow, Rschmertz, Russell E, Ryanaxp, S h i v a (Visnu), SBoyd415, SURIV, SWAdair, Saraphina,
Savantpol, Sbrools, Schumi555, Scienceboyroy, Scott0485, Scruss, Scuro, Shaddack, Shoy, Sifaka, Simishag, SimonP, Sjakkalle, Skittleys, Sly Marbro 03, Snarlinmonsta, Softlavender,
Someones life, Speddie2, Spellcast, Spoont3g, SpunkySkunk347, Spylab, Squigish, Squirdle Domino, Srikeit, Starstylers, Steven Zhang, Stitches OFire, Strattgr, Struthious Bandersnatch, Stw,
SubDural12, Svick, Syrthiss, Tai kit, TangParadise, Tccmod, Tdowling, Tea with toast, TeaDrinker, Tednor, TexasAndroid, The Anome, The Letter J, The Parting Glass, The Right Honourable,
The Thing That Should Not Be, The monkeyhate, TheEgyptian, Thepokeduck, Thingg, Thoric, Tiles, TimBentley, TinyHero55, Tirolion, Tlesher, Tony Sidaway, Tox, Transhumanist, Travelbird,
Travis1101, Traxs7, Trilobitealive, Trusslars, Tullie, USMarine1171, Use the force, Vanished user 03, Vaughan, Velvetron, Verkhovensky, Versus22, Vianegativa, Villahj Ideeut, Vlad,
Voyaging, WAjanus, WAvegetarian, WJBscribe, WVhybrid, Wayne Riddock, Wayne Slam, Weyes, Wg0867, Whirlingdervish, Whkoh, WikiHatch, WikiLeon, Wikibofh, Wikipediarules2221,
Will Beback Auto, William Avery, Wingsandsword, Wmahan, Wood Thrush, Woohookitty, WriterHound, Wtmitchell, XJamRastafire, Xe7al, Xrust, Xsn900, Yamamoto Ichiro, Yasunat, Yayay,
Yeliabrecneps, Yelyos, Yosri, Yurik, Zachbe, Zaphraud, ZooFari, Zzuuzz, طبلا يلع نسح, 1201 anonymous edits
Ataractive Source: http://en.wikipedia.org/w/index.php?oldid=387271861 Contributors: Bearcat, El3ctr0nika, طبلا يلع نسح
Cathinone Source: http://en.wikipedia.org/w/index.php?oldid=419633845 Contributors: Anypodetos, Arcadian, Astavats, AvicAWB, Avin, Beetstra, Bk0, C6541, Cacycle, CanisRufus,
ChemNerd, Chempedia, Chrumps, Cormacs, Cvf-ps, DanMS, Ddhix 2002, Eequor, El3ctr0nika, Eloil, Enix150, Fvasconcellos, Galaxiaad, Gentgeen, Gobonobo, Harbinary, Heah, Horse, Icairns,
IllusionalFate, InFairness, Jü, Lightmouse, Marcoisgang, MattKingston, Mutinus, Mysid, Niklo sv, Nirmos, Oskar71, PTSE, Portierrb, Pro crast in a tor, Rada, Rbaselt, Remember me,
Rhadamante, Rich Farmbrough, Rmky87, Russell E, S. Neuman, Sbrools, Selket, SimonP, That Guy, From That Show!, TimBentley, Tins128, UtherSRG, Vuo, Weyes, WriterHound, Xrust,
Zahnrad, 32 anonymous edits
Dextrorphan Source: http://en.wikipedia.org/w/index.php?oldid=427819677 Contributors: AlkaloidMan, AlphaMoron, Alvis, Anypodetos, Arm, Astavats, Barticus88, Bluemoose, C6541,
ClockworkLunch, Dcirovic, DemonicPartyHat, Derek.cashman, El3ctr0nika, Equazcion, Ettrig, Frank Lofaro Jr., Gabriel.Villeda, Gazeofsorrow, Gobonobo, Harbinary, Jolb, Kbh3rd, Malik
Shabazz, MattKingston, Montyburns105, Myrkkyhammas, Nirmos, Obrien2048, P2409, P33M, RJSampson, Rjwilmsi, Smack Shooter, Teutonic Tamer, Tommy2010, Wingedsubmariner, نسح
طبلا يلع, 24 anonymous edits
Dopamine reuptake inhibitor Source: http://en.wikipedia.org/w/index.php?oldid=417742711 Contributors: Academic Challenger, Agjchs, Arcadian, Atlanteanrecords, Axxaer, Bob247,
Bobbonew, C6541, Chris Capoccia, Cracked acorns, Cshay, Dspradau, Eequor, El3ctr0nika, Fuckyourmother, Gabethenerd, Galaxiaad, Grafen, Harbinary, Harjk, Jeepday, Jim Douglas, Jmax-,
Klausness, Lectonar, Lincher, Literaturegeek, McDogm, Mderezynski, Meodipt, N96, Nagelfar, Nihiltres, Nuklear, Parker007, Pwjb, R'n'B, Rmky87, Shcirnuh, Suboptimal Username,
Supergloom, Sweety Rose, Uthbrian, Woohookitty, Yrsukrutt, Zeppelin4life, طبلا يلع نسح, 112 anonymous edits
FX-1006A Source: http://en.wikipedia.org/w/index.php?oldid=394556636 Contributors: Bearcat, Malcolma, Rod57
GABA agonist Source: http://en.wikipedia.org/w/index.php?oldid=421425304 Contributors: Akane700, Arcadian, Bearcat, Jeffmcneill, Xezbeth, 6 anonymous edits
Melatonin Source: http://en.wikipedia.org/w/index.php?oldid=430151185 Contributors: 5 albert square, 5Q5, A314268, ABCD, AManWithNoPlan, AThing, Aarue, Ace Trigonometry,
Adambiswanger1, Addisonstrack, Adeez, Adrian.benko, Aenar, Africangenesis, Ahoerstemeier, Airplaneman, Alansohn, Alberto da Calvairate, Alex.tan, Alfie66, AltheaJ, Amigadave,
Andreas333, Angela, Anlace, Anna Lincoln, Anonywiki, AnthonyMBeck, Arcadian, Astavats, Attys, Avenged Eightfold, Awanta, B7T, BD2412, Badhotra, Beetstra, Benbest, BigChillRob,
Blc341, Bluemoose, Bobblehead, BorgQueen, Brighterorange, Bundlesaurus, Burleigh2, Byah108, C6541, Cacycle, Calyph, Casforty, Cat2020, Causa sui, Ccroberts, Celestialpillow, Chirality,
Chowbok, Chris Capoccia, Christian75, Clayoquot, Cliff smith, Codwiki, ColdFeet, Colin, Cometstyles, Cpawizard, Crazy-Chemist, CrazyChemGuy, Crl620, Custardninja, Cyrus Grisham, Dana
boomer, DanwWiki, DarkAdonis255, Darksun, DavidCary, Davidruben, Dcirovic, Ddhix 2002, Deflective, Delta G, Denovo1, Derek.cashman, Derengard, Dianadeister, Diannaa, Digitalsabre,
Dogface, Drmies, Drphilharmonic, Drugsaboutcom, Dunxian, E2eamon, ESkog, Edgar181, EdgeOfEpsilon, Ee1518, Eequor, El3ctr0nika, ElBenevolente, Electric goat, Elroch, Emezei,
Enchanter, Entropy, Enviroboy, EoGuy, Erik Garrison, Eubulides, EugeneZelenko, Ewick12, Exabyte, Fabrício Kury, Feelingtrees, Fenice, Ferred, Fishmaster, Flyguy649, FrenchIsAwesome,
Friedrich K., Froggydoo, Fuzheado, Fvasconcellos, Gabbe, Gaius Cornelius, Georgyo, Giftlite, Gioto, Glen, Gleng, GodGnipael, GraemeL, Guitarmankev1, Gwernol, Gzkn, H.G.Milliner,
Hagenb, Hal peridol, Halestock, Harold f, Heah, Henk Poley, Highnote, Hordaland, Hu12, IByte, Igoldste, Iman.saleh, Imnowei, ImperfectlyInformed, Imz, Ineuw, Inneedofedit, Insanephantom,
Intangir, Iothiania, Jacquepepin, James Matheson, Janopus, Jaredx420, Jellyfishing, Jjsimpsn, Jmh649, Jordantacannon, Jpgordon, Junkyardprince, Jvpugh, Karch, Karenca, Karl-Henner,
Kehrbykid, Kesh, KevinRoxis, Kghusker, Khalsaburg, Kingsize566, Kingturtle, Kkaq, Kmlong, Knwledgeispower2010, Koavf, Kuru, Kwamikagami, Lamiot, LicenseFee, Lights, LilHelpa, Lilac
Soul, Lilypink, Looxix, Lunarplexuses, M1ss1ontomars2k4, Maerk, Malljaja, MarkGallagher, Marksmany, Martin S Taylor, MastCell, Matt Lewis, Meco, Michael Bailes, Michael Devore,
Michaelmas1957, Mikael Häggström, Mike Rosoft, MinstrelOfC, Mjb, Mmortal03, Modulatum, Mpappolla, MrOllie, MrRumfoord, Mugaliens, Muspud2, MyFamilyDoctorMag, Naniwako,
Nascanner, Nbauman, Ndaniels, Nehrams2020, Neovita, Netheril96, NiOrtiga, Nick Taylor1, Nirmos, Nutriveg, OAC, Ochemchick, Oda Mari, OrangeDog, Orionus, Osterluzei, Overand,
PGRandom, Pashihiko, Pavlinmojca, Pgan002, Pierre-Alain Gouanvic, Pinkgirl15, Pipedreambomb, Pizza1512, Pol098, Politepunk, Pro crast in a tor, Pseudomonas, Pwjb, R'nway, R'son-W,
Reedy, Remember, RexNL, Rhansler, Rhombus, Rich Farmbrough, Rich257, Rjwilmsi, Roberdin, Roidroid, Rolano, Rollie, Ronz, Russell E, SDY, Saenger, Salamurai, SandyGeorgia, Sannse,
Sayeth, Sbrools, Scientizzle, Sethie, Seven of Nine, Shakinsam, Shidayu, Sigrunmelatonin, Silverback, Silverleaftree, Skomorokh, Sometime-science-editor, Stickee, Strider01, TheRhani,
Thorncrag, Tillbury, Timos55, Timwi, Torzsmokus, TrevMrgn, Tualha, Twsx, Uirauna, Use the force, Uthbrian, UtherSRG, Velvetron, Vexorg, Victimofleisure, Vov Abraxas, W2bh, WLU,
Walkerma, Wconnor3, Wereon, Wickey-nl, Winchelsea, Wjhonson, Wordsmith, WriterHound, Wrongful, Wtmitchell, Xyzzyplugh, Zmaddoc, Zoohouse, Роман Беккер, طبلا يلع نسح, 579
anonymous edits
Mephedrone Source: http://en.wikipedia.org/w/index.php?oldid=430353252 Contributors: 304steph304, 4twenty42o, ActionGaz, AdamNailor, Adamovince, Alansohn, Algebraist, Amajailbait,
Andrewjlockley, Anna Lincoln, Anomanus, Anthony Appleyard, Antrozous, Astavats, Aushulz, Bdog9121, Beetstra, Belovedfreak, Benjah-bmm27, Bgpaulus, Bihco, BorisAndDoris,
83
Article Sources and Contributors
Brandcrazy, Briantist, Brule Thesp, C6541, COSully, Campestris, Caponex, Casilingua, Catiline63, Cckkab, Chamal N, Chendy, Chris the speller, Christopher S Nicklin, ClamDip, Codemeister3,
Courcelles, Craignelson71, Craxyxarc, DARTH SIDIOUS 2, DC, DMacks, Daediter, DannyByrdUK, Darobian, Daveparker76, Daveydweeb, Dhidalgo, Digilante, Discospinster, DocWatson42,
Donirish, Download, Drmies, Dronetillshemoans, EALacey, Edgar181, El3ctr0nika, Element16, Emperor, Enix150, Excirial, Fastilysock, Favonian, Flibbertygibbet79, Foobaz, Fragglet, Frglee,
Fudgeandhayley, Funandtrvl, Gabbe, Gadget850, Gangstaell, Gargaj, Gesseg, Glane23, GordonRoss, GorillaWarfare, Grafen, Griffindd, Grim23, Hadrian89, Hajatvrc, Harbinary, HarlandQPitt,
Hartofdixie717, HexaChord, Hkarkus, Hoo man, Hortophilus, Husond, Immunize, Insanity Incarnate, Inverse.chi, IronGargoyle, ItalianChef, J.delanoy, JForget, Jaganjac, Jamesdowling1993,
Jaybilly, Jdogpisshmtherfker, Jinxed, Jla, John of Reading, Johnjacob1123, Jusdafax, JzG, Jü, KTo288, Kkpb8122, Kocio, Kotakkasut, Krashlandon, KrazyPhrick, Kubigula, Kwenchin,
LDNAware, Lebelu2007, MICHAELCLIENS, MMS2013, Malick78, Malkuth101, Managermerrill, Mandarax, Markoff Chaney, Materialscientist, McSly, Meaghan, Mefrit, Meodipt,
Meowinwales, Mere Mortal, Metallion, Methecooldude, Miserlou, MoondyneAWB, Motorbase, MultiTaskCounsler, MuzikJunky, Mygerardromance, Mylenium75016, N3ak, N419BH, N5iln,
Nabokov, Nach0king, Nagelfar, NawlinWiki, Nelsonoah, Neo139, NewEnglandYankee, Niba07, Nick Cooper, Nickin, Nirmos, Nono64, Noq, Orphan Wiki, Oswaldmuesli, P0mbal, Pdcook,
Pearlised, Phantomsteve, Pianoplonkers, Pmarci, Pokraka, Pontificalibus, Postlewaight, Poul818, Praefectorian, Pseudomonas, Psilfy, Pythoness Mar, Rbaselt, Rcdrugs com, Rd232, Reach Out to
the Truth, Relum Evets, Richard75, Riemann Zeta, Ring0, Rjwilmsi, Rmosler2100, Rockhyrax, Ronhjones, Rooster82, RoyBoy, SDC, STBAT42569, Samsbase, Sasata, Scapler, Scientist111,
Scrodillyotum, Seaphoto, Sinned, Slowpoke, Smartse, Soap, Spark1234as, SpikeToronto, Spitfire19, Srbmarine, Ssupers, Stoned420, Tacitus2000, Thatguyflint, The chemistds, Theastrodude,
Therainmakere, Thumperward, Tide rolls, Tillbury, Tkynerd, Tommy2010, Torqueing, Tortfeasor91, Totakeke423, ToxicOranges, UTIOMAS, Ulner, Vertikar, VictorianMutant, Vince7133,
Welsh, Wildespace, Willking1979, Wknight94, Wostr, Xris0, Yngvadottir, Yobol, Zengar Zombolt, 984 anonymous edits
Norepinephrine releasing agent Source: http://en.wikipedia.org/w/index.php?oldid=357746411 Contributors: El3ctr0nika, 1 anonymous edits
Norepinephrine reuptake inhibitor Source: http://en.wikipedia.org/w/index.php?oldid=424857568 Contributors: Acdx, Agjchs, Agwoodliffe, Arcadian, Aytrus, Cbh, Chowbok, Chris
Capoccia, Delldot, Dppowell, Eequor, El3ctr0nika, Epolk, Galaxiaad, Harbinary, JaGa, Jay Litman, Jmax-, Jni, Katpjotr, Magister Mathematicae, Meodipt, Mgiganteus1, Nihiltres, Nuklear, Q
uant, RxS, Scientizzle, Shadowlapis, Simman2, Sonjaaa, Wadems, West.andrew.g, Wg0867, Woohookitty, Xabian40409, Yrsukrutt, طبلا يلع نسح, 25 anonymous edits
Propylhexedrine Source: http://en.wikipedia.org/w/index.php?oldid=429774764 Contributors: AdjustShift, Aeons, Alansohn, All.ya.little.triksters, B, Beetstra, Belovedfreak, Bigd063,
Bluemoose, C6541, Ceyockey, Colonies Chris, DMacks, Ddhix 2002, DemoIXI, Eduardoporcher, El3ctr0nika, Eyeballcancer, Fuzzform, Fvasconcellos, Gazeofsorrow, Gonzonoir, Gurch,
Heathhunnicutt, Hoffmeier, Jacobso4, JamesAM, Jerry guru, Jkl sem, Joie de Vivre, Jü, Killbuddy, Ledcraft, Lisapollison, Mike mathews, Night Gyr, Nihiltres, Nuklear, Onco p53, PamD, Rada,
Ryanaxp, Selket, Semilanceata, Skysmith, St3vo, Stirfryguy, Thesmallprint189, X1987x, طبلا يلع نسح, 124 anonymous edits
Psychoanaleptic Source: http://en.wikipedia.org/w/index.php?oldid=414649091 Contributors: Arcadian, Arent, Bearcat, Diligent, Malcolma, Patxi lurra, Quux0r, Rünno, Seven of Nine, The
Founders Intent, 2 anonymous edits
Selective serotonin releasing agent Source: http://en.wikipedia.org/w/index.php?oldid=376955510 Contributors: El3ctr0nika
Serotonin releasing agent Source: http://en.wikipedia.org/w/index.php?oldid=406245765 Contributors: El3ctr0nika, Rich Farmbrough, Rjwilmsi
Serotonin reuptake inhibitor Source: http://en.wikipedia.org/w/index.php?oldid=417863107 Contributors: Alatari, Anthony Appleyard, Arcadian, Belovedfreak, C6541, Edgar181,
El3ctr0nika, Fratrep, Grafen, Meodipt, Rich Farmbrough, Scientizzle, Woohookitty, Yrsukrutt, 12 anonymous edits
84
Image Sources, Licenses and Contributors
Image Sources, Licenses and Contributors
Image:Adenosin.svg Source: http://en.wikipedia.org/w/index.php?title=File:Adenosin.svg License: Public Domain Contributors: User:NEUROtiker
file:Amphetamine-2D-skeletal.svg Source: http://en.wikipedia.org/w/index.php?title=File:Amphetamine-2D-skeletal.svg License: Public Domain Contributors: User:Chesnok
file:Amphetamine-3d-CPK.png Source: http://en.wikipedia.org/w/index.php?title=File:Amphetamine-3d-CPK.png License: Creative Commons Attribution-Sharealike 2.5 Contributors:
User:Sbrools
File:Yes check.svg Source: http://en.wikipedia.org/w/index.php?title=File:Yes_check.svg License: Public Domain Contributors: User:Gmaxwell, User:WarX
Image:X mark.svg Source: http://en.wikipedia.org/w/index.php?title=File:X_mark.svg License: GNU Free Documentation License Contributors: Abnormaal, DieBuche, Gmaxwell, Kilom691,
Kwj2772, MGA73, Mardetanha, Penubag, Pseudomoi, WikipediaMaster, 1 anonymous edits
File:Rational scale to assess the harm of drugs (mean physical harm and mean dependence).svg Source:
http://en.wikipedia.org/w/index.php?title=File:Rational_scale_to_assess_the_harm_of_drugs_(mean_physical_harm_and_mean_dependence).svg License: Public Domain Contributors:
User:Apartmento2
File:Amphetamine-2D-skeletal.svg Source: http://en.wikipedia.org/w/index.php?title=File:Amphetamine-2D-skeletal.svg License: Public Domain Contributors: User:Chesnok
Image:Methamphetamines.PNG Source: http://en.wikipedia.org/w/index.php?title=File:Methamphetamines.PNG License: Public Domain Contributors: Original uploader was
Shoyrudude555 at en.wikipedia
file:S-Cathinone.svg Source: http://en.wikipedia.org/w/index.php?title=File:S-Cathinone.svg License: Public Domain Contributors: Ayacop, Wickey-nl
file:Cathinone-3d-CPK.png Source: http://en.wikipedia.org/w/index.php?title=File:Cathinone-3d-CPK.png License: Creative Commons Attribution-Sharealike 2.5 Contributors: User:Sbrools
Image:Cathinone-3d-sticks.png Source: http://en.wikipedia.org/w/index.php?title=File:Cathinone-3d-sticks.png License: Creative Commons Attribution-Sharealike 2.5 Contributors:
User:Sbrools
file:Dextrorphan.svg Source: http://en.wikipedia.org/w/index.php?title=File:Dextrorphan.svg License: Public Domain Contributors: User:Harbin
file:Dextrorphane 3d.gif Source: http://en.wikipedia.org/w/index.php?title=File:Dextrorphane_3d.gif License: Free Art License Contributors: User:C6541
Image:Dopamine.png Source: http://en.wikipedia.org/w/index.php?title=File:Dopamine.png License: Public Domain Contributors: C.Löser, Edgar181, NEUROtiker
File:Gamma-Aminobuttersäure - gamma-aminobutyric acid.svg Source: http://en.wikipedia.org/w/index.php?title=File:Gamma-Aminobuttersäure_-_gamma-aminobutyric_acid.svg License:
Public Domain Contributors: User:NEUROtiker
file:Melatonin2.svg Source: http://en.wikipedia.org/w/index.php?title=File:Melatonin2.svg License: Public Domain Contributors: User:NEUROtiker
file:Melatonin-3d-CPK.png Source: http://en.wikipedia.org/w/index.php?title=File:Melatonin-3d-CPK.png License: Creative Commons Attribution-Sharealike 2.5 Contributors: User:Sbrools
Image:Walgreens Melatonin-2010-20-07.jpg Source: http://en.wikipedia.org/w/index.php?title=File:Walgreens_Melatonin-2010-20-07.jpg License: Creative Commons Attribution 2.0
Contributors: User:Zoohouse
file:Mephedrone-2D-skeletal.png Source: http://en.wikipedia.org/w/index.php?title=File:Mephedrone-2D-skeletal.png License: Public Domain Contributors: Ben Mills
file:4-MMC_3D.gif Source: http://en.wikipedia.org/w/index.php?title=File:4-MMC_3D.gif License: Public Domain Contributors: C6541
File:Mephedrone seizures.png Source: http://en.wikipedia.org/w/index.php?title=File:Mephedrone_seizures.png License: Creative Commons Attribution-Sharealike 3.0 Contributors:
User:Smartse
File:Mephedrone-enantiomers-Spartan-HF-3-21G-3D-balls.png Source: http://en.wikipedia.org/w/index.php?title=File:Mephedrone-enantiomers-Spartan-HF-3-21G-3D-balls.png License:
Public Domain Contributors: Ben Mills
File:Mephedrone metabolism.png Source: http://en.wikipedia.org/w/index.php?title=File:Mephedrone_metabolism.png License: Public Domain Contributors: User:Edgar181
File:Mephedrone-synthesis-scheme-2D-skeletal-B.png Source: http://en.wikipedia.org/w/index.php?title=File:Mephedrone-synthesis-scheme-2D-skeletal-B.png License: Public Domain
Contributors: Ben Mills
Image:4mmc.png Source: http://en.wikipedia.org/w/index.php?title=File:4mmc.png License: Public Domain Contributors: DEA
Image:Epinephrine structure with descriptor.svg Source: http://en.wikipedia.org/w/index.php?title=File:Epinephrine_structure_with_descriptor.svg License: Creative Commons
Attribution-Sharealike 3.0 Contributors: User:Acdx
Image:Modern NET ligands.svg Source: http://en.wikipedia.org/w/index.php?title=File:Modern_NET_ligands.svg License: Public Domain Contributors: User:Edgar181
file:Propylhexedrine.svg Source: http://en.wikipedia.org/w/index.php?title=File:Propylhexedrine.svg License: Public Domain Contributors: User:Fvasconcellos
file:Propylhexedrine.gif Source: http://en.wikipedia.org/w/index.php?title=File:Propylhexedrine.gif License: Public Domain Contributors: User:C6541
Image:Fenfluramine.png Source: http://en.wikipedia.org/w/index.php?title=File:Fenfluramine.png License: Public Domain Contributors: User:Hoffmeier
Image:Serotonin (5-HT).svg Source: http://en.wikipedia.org/w/index.php?title=File:Serotonin_(5-HT).svg License: Public Domain Contributors: User:NEUROtiker
85
License
License
Creative Commons Attribution-Share Alike 3.0 Unported
http:/ / creativecommons. org/ licenses/ by-sa/ 3. 0/
86