* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Genotoxic evaluation of olanzapina (Zyprexa Zydis®) in mouse
Survey
Document related concepts
Transcript
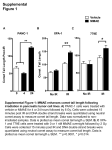
57º Congresso Brasileiro de Genética Resumos do 57º Congresso Brasileiro de Genética • 30 de agosto a 2 de setembro de 2011 Centro de Convenções do Hotel Monte Real Resort • Águas de Lindóia • SP • Brasil www.sbg.org.br - ISBN 978-85-89109-06-2 88 Genotoxic evaluation of olanzapina (Zyprexa Zydis®) in mouse (Rattus norvegicus) by the comet assay Ferreira, TS1, Sales, NK1 Gonçalves, MW1, Carvalho, WF1, Bernini, RB1,3, Da Cruz, AD1,2. 1 Núcleo de Pesquisas Replicon – Pontifícia Universidade Católica de Goiás. 2 Programa de Pós-Graduação em Biologia Celular e Molecular - Universidade Federal de Goiás. 3 Pax Clínica Psiquiátrica – Instituto de Neurociência. [email protected] Keywords: Comet assay, Rodents, Olanzapina, Genotoxicity, Mutagenicity. Olanzapine (Zyprexa Zydis®) is indicated for the acute treatment and maintenance of schizophrenia and other psychoses where symptoms are prominent. This study aimed to evaluate the mutagenic potential of olanzapine in rodents (Rattus norvegicus) by comet assay. We used eight rodents being 4 females and 4 males aged 45 days, from CETAS (Screening Center for Wild Animals) – IBAMA/GO, kept them at 22 ° C ± 1 ° C in a light/dark cycle of 12h/12h (6h - 18h) with ad libitum access to food and water. The animals were divided into four groups of two individuals (one male and one female) for each treatment. During a period of 38 days the drug was administered (orally). The control group received only glucose solution (Glucose 5%), Group 1 (4 mg/kg), Group 2 (6 mg/kg) and Group3 (10mg/kg) received olanzapine. The comet assay was performed as described by Silva et al. (2000) with few modifications. The animals were anesthetized with ether, and sacrificed by cervical dislocation. The cell suspensions obtained from bone marrow were rapidly transferred to ependorff and protected from light. The slides were prepared with a precoat “Normal Melting” agarose (1.5%). We collected 20 µL of cell suspension of each individual and diluted in 120 µL of “Low Melting Point” agarose 0.5% (37 º C). This mixture was pipetted on the slides with the pre-coat and covered with a coverslip, and placed in the refrigerator at 4 °C for two minutes so that there was the solidification of the material. After this time the coverslips were removed and the slides were then immersed in lysis buffer for 24 hours. Then, it was followed by the electrophoresis for 25 minutes at 25 volts and 300 mAps. The alkalinity was interrupted with neutralizing buffer (pH 7.4). The DNA was then flushed with 25 µL of ethidium bromide (10 ng / µl). Nuclei were visualized using fluorescence microscopy, “software” ISIS®, 10X objective. Two slides were analyzed per individual with 50 cells each, totalizing 100 cells per individual. The analysis of the nucleoids was performed using the “Comet Score” version 1.5. For the statistical analysis used the “t” test, significance level of 5%. The group1 had an average length of the comet of 34.99 + 14.57 arbitrary units (AU); group2 + 34.75 13.84 (AU); group3 + 29.93 12.21 (AU) and the control group an average length of the comet of 36, 42 + 8.52 (AU). The “t” test revealed no significant differences between groups when compared with controls (p> 0.05). We can infer that the drug olanzapine did not result in genomic damage in the animals studied. Financial support: CNPq