* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download chapter24a
Oesophagostomum wikipedia , lookup
Schistosomiasis wikipedia , lookup
Neonatal infection wikipedia , lookup
Hepatitis C wikipedia , lookup
Trichinosis wikipedia , lookup
Human cytomegalovirus wikipedia , lookup
Ebola virus disease wikipedia , lookup
Tuberculosis wikipedia , lookup
West Nile fever wikipedia , lookup
Leptospirosis wikipedia , lookup
Marburg virus disease wikipedia , lookup
Whooping cough wikipedia , lookup
Swine influenza wikipedia , lookup
Middle East respiratory syndrome wikipedia , lookup
Hepatitis B wikipedia , lookup
Henipavirus wikipedia , lookup
Herpes simplex virus wikipedia , lookup
Lymphocytic choriomeningitis wikipedia , lookup
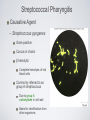
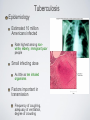
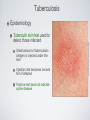
Respiratory System Infections Chapter 24 Respiratory System Infections Encompass enormous variety of illnesses Trivial to fatal Divided into infections of Upper respiratory Head and neck Uncomfortable but generally not life threatening Lower respiratory Chest More serious Can be life-threatening Particularly in the immunocompromised Normal Flora Nasal cavity, nasopharynx and pharynx colonized by numerous bacteria Other sites are sterile Numerous classes of organisms are present from aerobes to anaerobes Conjunctiva commonly have no bacteria Organisms that do invade are swept into the nasolacrimal duct (tear duct) and nasopharynx Streptococcal Pharyngitis Symptoms Characterized by Difficulty swallowing Fever Red throat with pus patches Enlarged tender lymph nodes Localized to neck Most patients recover uneventfully in approximately a week Streptococcal Pharyngitis Causative Agent – Streptococcus pyogenes Gram-positive Coccus in chains β hemolytic Complete hemolysis of red blood cells Commonly referred to as group A streptococcus Due to group A carbohydrate in cell wall Basis for identification from other organisms Streptococcal Pharyngitis Pathogenesis due to numerous virulence factors Streptolysin Protein G Complications of infection can occur during acute illness Scarlet fever Acute glomerulonephritis Acute rheumatic fever Treatment Penicillin is antibiotic of choice Common Cold Symptoms Malaise Scratchy mild sore throat Runny nose Cough and hoarsness Nasal secretion Initially profuse and watery Later, thick and purulent No fever Unless complicated with secondary infection Symptoms disappear in about a week Common Cold Causative Agent 30% to 50% caused by rhinoviruses More than 100 serotypes of rhinoviruses Non-enveloped Single-stranded RNA genome Common Cold Pathogenesis Virus attach to specific receptors on respiratory epithelial cells and multiply in cells Large number of viruses released from infected cells Injured cells cause inflammation which stimulates profuse nasal secretion, sneezing and tissue swelling Infection is halted by inflammatory response, interferon release, and immune response Infection can extend to ears, sinuses and lower respiratory tract before stopping Treatment is supportive with OTC medications Whooping Cough Aka: Pertussis Symptoms Runny nose followed by bouts of uncontrollable coughing Termed paroxymal coughing Severe cough can cause rupture of small blood vessels in the eyes Coughing spasm followed by characteristic “whoop” Sound made by the forceful inspiration of air Vomiting and seizure may occur Whooping Cough Causative Agent – Bordetella pertussis Small Encapsulated Strictly aerobic Gram-negative Bacillus Does not survive long periods outside the host Whooping Cough Pathogenesis Enters respiratory tract with inspired air and attaches to ciliated cells Organism colonizes structures of the upper and lower respiratory tract Mucus secretion increases which causes ciliary action to decrease Cough reflex is only mechanism for clearing secretions Whooping Cough Video on web page Whooping Cough Pathogenesis – B. pertussis produces numerous toxic products Pertussis toxin - A-B toxin B portion attaches to cell surface A portion enters cell and inactivates regulation of cAMP Causes increased mucus formation Decreases phagocytic killing Invasive adenylate cyclase Increases production of cAMP Increased mucus formation Whooping Cough Epidemiology Spreads via infected respiratory droplets Most infectious during runny nose period Number of organisms decrease with onset of cough Classically disease of infants Milder forms are seen in older children and adults Often overlooked a persistent cold Fosters transmission Whooping Cough Prevention Vaccine directed at protection of infants Prevents disease in 70% of individuals Pertussis vaccine combined with diphtheria and tetanus toxoids (DPT) Injections given at 6 weeks, 4, 6,and 18 months, 5 years, and now recommended for 12 year olds Treatment Erythromycin is effective at reducing symptoms if given early Tuberculosis Causative Agent Symptoms Chronic illness Mycobacterium tuberculosis (and M. bovis in AIDS) Symptoms include Gram-positive cell wall Slight fever with night sweatsSlender bacillus Progressive weight loss Chronic productive cough Acid fast due to mycolic acid in cell wall Slow growing Sputum often blood streaked Generation time 12 hours or more Resists most prevention methods of control Tuberculosis Pathogenesis Usually contracted by inhalation of airborne organisms Bacteria are taken up by pulmonary macrophages in the lungs Resists destruction within phagocyte Organism prevents the fusion of phagosome with lysosomes; allows multiplication in protected vacuole Tuberculosis Pathogenesis Organisms are carried to lymph nodes About 2 weeks post infection intense immune reaction occurs Macrophages fuse together to make large multinucleated cell Macrophages and lymphocytes surround large cell and form a tubercle of connective tissue This is an effort to wall off infected tissue Activated macrophages release into infected tissue Causes death of tissue resulting in formation of “cheesy” material (biofilm) Tuberculosis Epidemiology Estimated 10 million Americans infected Rate highest among nonwhite, elderly, immigrant poor people Small infecting dose As little as ten inhaled organisms Factors important in transmission Frequency of coughing, adequacy of ventilation, degree of crowding Tuberculosis Epidemiology Tuberculin skin test used to detect those infected Small amount of tuberculosis antigen is injected under the skin Injection site becomes red and firm if infected Positive test does not indicate active disease Tuberculosis Prevention Treatment Vaccination for tuberculosis Antibiotic treatment is given in cases of active widely used in many parts tuberculosis of the world Two or more medications are given together to reduce potential antimicrobial resistance Vaccine known as Bacillus of Calmette and Guérin Antimicrobials include Rifampin and Isoniazid (INH) BCG derived from Mycobacterium bovis Gives weak, partial immunity against tuberculosis Both target actively growing organisms and metabolically inactive intracellular organisms Therapy is pronged, lasting at least 6 months Vaccine not given in United Multi-drug resistant strains have States because it arisen in the U. S. and Russia that eliminates use of tuberculin have spread to other countries test as diagnostic tool Seasonal Influenza Symptoms Influenza Type A Short incubation period Averaging 2 days Headache Fever Muscle pain Dry cough Acute symptoms abate within a week Cough, fatigue and generalized weakness may linger Seasonal Influenza Causative Agents Influenza A virus Belong to orthomyxovirus Single-stranded RNA genome Genome divided into 8 gene segments Spiked envelope H spike – hemagglutinin (subtypes H1-H16) Aids in attachment Only H1, H2 and H3 viruses circulate in humans N spikes – neuraminidase (subtypes N1-N9) Cleaves H protein to allow fusion of viral and cellular membranes (i.e., entry into the cell) Requires cellular enzyme trypsin to facilitate infection Influenza B & C viruses only circulate in humans Influenza A Transmission Cycle Adaptation/ reassortment with swine influenza viruses Circulates with limited pathology Transmission to domestic fowl Transmission to humans Pathogenesis Seasonal Influenza Acquired through inhalation of infected respiratory secretions Virus attaches to host cells via hemagglutinin spikes Once attached viral envelope fuses with host membrane, leading to viral replication within the cell Mature viruses bud from host cell Budding allows mature virus to pick up envelope Infected cells die and slough off Host immunity quickly controls viral spread Anti-HA neutralizing IgG is protective Mortality rate is low However, hundreds of thousands or millions of people are infected each year in the U. S. On average, about 30,000 Americans, mostly elderly and very young children, die from influenza each year Seasonal Influenza Epidemiology Outbreaks occur in United States every year Vaccines are formulated months in advance using prominent circulating strains 2010-2011 vaccine strains Type / Geographic origin / Strain/ Year isolated (H & N genes) A/California/7/2009 (H1N1)-like (the same strain as was used for 2009 H1N1 monovalent vaccines) A/Perth/16/2009 (H3N2)-like B/Brisbane/60/2008-like Pandemics occur periodically Most famous pandemic of 1918 (Spanish flu) Spanned the globe in 9 months Pandemics have higher than normal morbidity Seasonal Influenza Epidemiology Spread caused by major antigenic changes Antigenic drift Consists of amino acid changes in spikes (point mutations) Particularly hemagglutinin Antigenic shift Represent more dramatic changes Virus strains are drastically antigenically different from previous strains, importantly in the hemagglutinin Changes minimize effectiveness New virus comes from genetic reof immunity to previous strains assortment Ensures enough susceptible people are available for continued virus survival Occurs when two different viruses infect a cell at the same time Genetic mixing results in new virus that is often more virulent Seasonal Influenza Seasonal Influenza Prevention and Treatment Vaccine can be 80% to 90% effective New vaccine required every year Due to antigenic drift Antiviral medications are 70% to 90% effective Include amantadine, rimantadine, and Tamiflu Must be taken early Not a substitute for vaccine Avian Influenza There are hundreds, if not thousands, of influenza A viruses circulating in nature Seasonal influenza occurs from mammalian viruses Pigs in SE Asia are frequently a source of these viruses New reassortants arise every year, but most are not pathogenic to humans Avian influenza viruses routinely circulate among wild birds Some species can be infected without conspicuous pathology These species often carry the viruses along migratory routes, exposing other birds Avian Influenza Most avian influenza viruses are highly inefficient at infecting humans However, some cultures have domestic birds and pigs in close periodomestic proximity This practice increases the chance of Reassortment with mammalian influenza viruses 1957, 1967 pandemic strains were reasortant mammalian viruses with avian segments (antigenic shift) Emergence of mutant avian strains that can infect humans 1918 pandemic strain was an avian virus that adapted to efficient human to human transmission (antigenic drift) Avian Influenza Rescue of the 1918 pandemic strain Virology did not exist in 1918 The virus could not be isolated, thus went extinct when the pandemic ended In 2005 a group resurrected the 1918 strain from bodies buried in Alaskan permafrost Viral genome sequencing indicated it was an avian influenza A virus It was also infectious... Avian Influenza Tumpey et al., 310:77. 2005 Lungs from Mice Infected with Rescued 1918 H1N1 Pandemic Virus 1918 Strain 1918 Strain 1918 Strain with Texas 1991 H segment 1918 Strain 1918 Strain(HN)/Texas 1991 Strain hybrid Texas 1991 strain (control) Avian Influenza Feature SI1 H5N1 AI2 1918 H1N13 Transmission efficiency High Very low/none High Replication site Upper and lower respiratory tract Lower RT only Likely upper & lower RT Viral CPE4 Limited Substantial Substantial Immunopathology Limited Substantial Substantial Kills embyronated chicken eggs? No Yes Yes Requires trypsin for infection of cell cultures? Yes No No Vaccine Yes No N/A Fatality rate 0.03% (U.S.) 57% (global) About 1-2% (U.S) Demographic Young children, elderly Young adults Young adults 1 Seasonal influenza 2 Currently circulating H5N1 avian influenza virus 3 Rescued 1918 pandemic H1N1 avian influenza virus 4 Cytopathic effect (damage directly caused by the virus) Red - more pathogenic feature Green - less pathogenic feature Hantavirus (Cardio)Pulmonary Syndrome Symptoms Early symptoms Causative Agents Hantaviruses Fever Includes Sin Nombre virus found in initial outbreak Muscle ache Especially in the lower back Nausea and vomiting Diarrhea Later symptoms Unproductive cough Belong to bunyavirus family Single-stranded RNA genome Divided into 3 segments Enveloped Causes apathogenic, lifetime infection in rodent hosts Increasing shortness of breath Death in humans is caused by cardiac failure from severe hypotension Capillary leak syndrome in lungs Shock and death Hantavirus Pulmonary Syndrome Pathogenesis Enters body via inhalation of dust contaminated with urine, feces and saliva of infected rodents Viremia Mechanism unknown Carried throughout body Infects capillary endothelial cells Inflammation causes capillaries to leak fluid into lungs Causes hypoxia and hypotension Cardiac shock and death occurs in over 36% of patients Hantavirus Pulmonary Syndrome Hantavirus disease is a T cell cytokine-mediated immunopathology TNF IFNγ Normal Hantavirus Infection Interleukin-1 Interleukin-2 Lymphotoxin Proinflammatory cytokines •Immune cell infiltrates •No viral damage to the lung epithelium Hantavirus Pulmonary Syndrome Epidemiology Emerging disease due to recent discovery Most cases in United States occur west of Mississippi River Principally caused by Sin Nombre virus Deer mice (Peromyscus maniculatus) are the reservoir Outbreaks causality with increase rodent populations Many, if not all, infected deer mice become persistently infected Person-to-person transmission does not occur Notable exception: Andes hantavirus in South America (Argentina, Chile) Hantavirus Pulmonary Syndrome Prevention and Treatment Prevention is directed towards minimizing exposure Keep pet and human food in containers Maximal ventilation when cleaning mouse droppings Mop with disinfectant NEVER use brooms or vacuums Wear N-100 HEPA-filtered mask Lethal traps and poisons to decrease rodent population No effective antiviral treatment (nor would it work anyway) Treatment limited to supportive care Extracorporeal membrane oxygenation (ECMO)