* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download File
Survey
Document related concepts
Transcript
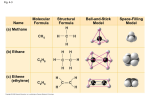
12th grade – March 15, 2016 Who would like to lead us in Our prayer today? News: Math tests are MONDAYS Science tests are FRIDAYS Quizzes are any days TESTS may need to be moved if we are not quite ready…but that is the exception. You need to check the weebly every night BEFORE you start your HW!!!!! Important March Dates: March 4th Spring fling/dance for 6th-12th grade/4-6 pm March 7th and 8th Sophia Academy closed March 9th Reconciliation available for students March 11th Midterm Progress Reports sent home March 16th Conference Day - No school for students Students in grades 5 through 12 must be present for their conference. March 24th Professional Development Day - No school for students ~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~ March 25th Good Friday - Sophia Academy closed March 28th Easter Monday - Sophia Academy closed PHYSICAL SCIENCE TAKE NOTES IN YOUR SCIENCE SPIRAL Homework was: RQ 11-20 REVIEW QUESTIONS 1. What are some uses of hydrocarbons? Hydrocarbons are used in to make plastics, as petroleum and as natural gases…FLAVORINGS, POLYMERS, FUELS, MEDICINES, AGRICULTURE, AND MORE… 2. How do two structural isomers differ from each other? Structural isomers have different arrangements of their carbon atoms, but the number of carbon atoms they each have is the same. 3. How are two structural isomers similar to each other? Structural isomers have identical chemical formulas. 4. What physical property of hydrocarbons is used in fractional distillation? Boiling points of hydrocarbons are used for fractional distillation. 5. To how many atoms is a saturated carbon atom bonded? A carbon atom in a saturated hydrocarbon is bonded to 4 atoms. 6. What is the difference between a saturated hydrocarbon and an unsaturated hydrocarbon? Saturated hydrocarbons have only single bonds. Unsaturated hydrocarbons have multiple bonds. 7. How many multiple bonds must a hydrocarbon have in order to be classified as unsaturated? A hydrocarbon must have at least one multiple (double or triple) bond to be classified as unsaturated. 8. Aromatic compounds contain what kind of ring? Aromatic compounds contain a 6-membered ring system called a benzene ring. 9. What is a heteroatom? A heteroatom is any atom other than carbon or hydrogen in an organic molecule. 10. Which heteroatom is characteristic of an alcohol? An oxygen atom bonded to a hydrogen atom and a carbon atom. 11-20 11. Why are low-formula-mass alcohols soluble in water? Small alcohols are soluble in water because they have polar oxygen-hydrogen bonds similar to water. Like dissolves like. 12. What distinguishes an alcohol from an ether? An alcohol has a hydroxyl (oxygen bonded to hydrogen) group, but an ether contains an oxygen atom bonded to two carbon atoms. 13. Which heteroatom is characteristic of an amine? Nitrogen is found in all amines. 14. Do amines tend to be acidic, neutral, or basic? Amines tend to be basic. The lone electron pair located on nitrogen atoms makes them basic because this lone pair is able to accept a hydrogen ion. 15. Are alkaloids found in nature? An alkaloid is a naturally occurring amine. 16. Which elements make up the carbonyl group? Carbon and oxygen make up a carbonyl group. 17. How are ketones and aldehydes related to each other? How are they different from each other? Both contain carbonyl groups. Ketones have the carbonyl carbon bonded to two adjacent carbon atoms, but aldehydes have the carbonyl carbon bonded to either one hydrogen and one carbon or to two hydrogens. 18. How are amides and carboxylic acids related to each other? How are they different from each other? Amides and carboxylic acids both contain carbonyl groups. Amides have the carbonyl carbon bonded to a nitrogen atom, but carboxylic acids have the carbonyl carbon bonded to a hydroxyl group. 19. What happens to the double bond of a monomer participating in the formation of an addition polymer? One of the bonds in the multiple bond of a monomer opens to form a new bond with a neighboring monomer molecule. 20. What is released in the formation of a condensation polymer? Upon formation of a condensation polymer, a small molecule (i.e., water or hydrochloric acid) is released from each monomer. Test is Friday – on RQ and RATs – not open notes – you must be able to do matching and fill in the blank with a word bank. http://www.conceptualacademy.com/textbook/conceptual-physical-science I HAVE SOME HW 4 U HW = RATS 1-10 AND START STUDYING 4 TEST! NOT OPEN NOTES!! START ON HW NOW – GO! PreCalculus Glogs due Monday…ELSE = 0…BEGINNING MARCH 14, 2016. Remember – all odds must be done then checked then corrected if wrong. HW WAS: Cw=>HW 3/11 = Pi graph: HW CHECK NOW = TO ME WITH GLOG 1 MINUTE = GO When is this used in real life?? Today 3/15 = 8.3 factoring trig equations Page 542-543 Ex.7 Think of factoring: - GCF** - “foil” 3 sin2 x – sinx – 2 = 0 How many terms? 3 3sin2 x – sinx – 2 = 0 Let ‘u’ = sin x 3u2 – u – 2 = 0 ?gcf? no 3u2 – u – 2 = 0 ?foil? (3u +- ??) (u +- ??) 2 1 1 2 (3u +2) (u -1) 3u2 - 3u + 2u -2 3u2 -1u -2 = yes right!!! Hw = Tuesday = due Thursday 3/17: Find a career where trig is used daily… Write a 1 page paper on this… Identify - job - Education needed Type of company Salary beginner mid-range top-level How is trig used – be specific and detailed *Do a good job *cite all sources *Grammar spelling complete sentences *Make sense and do not need corrections. *test grade…make it good. Cw=>HW 3/11 = Pi graph: PI MATH ART What you need: Markers in different colors – how many colors?? 0123456789 = 10 colors + black(.) or all black. Graph paper – or do it on your computer in Excel…be sure to use the same color for each same number…for example – all 3’s should be the same color…all 1’s should be the same color…etc. Math-crazed (or not) kids HOW TO MAKE A PI SKYLINE Each building represents a number in pi. Color in the number of squares on the graph paper that correspond to each digit of pi… Make a black dot to represent the decimal point… Fill in columns of squares for as many digits as will fit on one piece of paper in landscape mode. Graph ‘3’ as 3 squares – graph a ‘9’ as 9 squares… Remember – FOR ALL CLASSES – to note every 30 minutes!!! option 1 = do 30 min on each class hw start with math - then you must move on to another class…then come back if you need to… option 2 = there is no option 2. ====================================== OTHER 12th grade HW IS LISTED BELOW: CONFERENCES TOMORROW = NO PJ’S LIT = ROCKET BOYS…STUDY – TEST Thursday* US HISTORY = RELIGION = pray & rice box & project/essay on your cathedrals due 3/14 ECON = TAKE NOTES ON MARKET STRUCTURES* Spanish – research your next location … FAMILY TREE* http://spacetracks.tumblr.com/post/71651919119/spirographof-the-universe-the-orbital-dance-of MATEO SAYS: Go to that cool link - DUH!!!