* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Chapter 4 ppt - Issaquah Connect
Survey
Document related concepts
Transcript
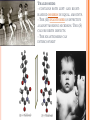
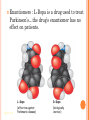
CHAPTER 4: CARBON CHEMISTRY Organic chemistry studies carbon compounds; the backbone of biological macromolecules When C forms four covalent bonds, its hybrid orbitals create a tetrahedral shape. When 2 C’s are joined by a double bond, a flat molecule forms The electron configuration of carbon gives it covalent compatibility with many different elements. What are some possibilities of molecules or compounds that could form with the following molecules? Figure 4.4 Hydrogen Oxygen Nitrogen Carbon (valence = 1) (valence = 2) (valence = 3) (valence = 4) H O N C CARBON BONDING Carbons readily bond with each other; they form single, double, triple, or quadruple bonds Name and Comments Molecular Structural Formula Formula H (a) Methane CH4 H C H H (b) Ethane C2H6 H H H C C H H H (c) Ethene Figure 4.3 A-C (ethylene) C2H4 H H C C H H Ball-andStick Model SpaceFilling Model ISOMERS Isomers: compounds with same molecular formula but different structures & properties 1. Structural: differ in covalent arrangements of atoms or double bond locations 2. Geometric (stereoisomers): differ in spatial arrangements; have cis and trans arrangements 3. Enantiomers: mirror-image molecules (a) Structural isomers (b) Geometric isomers H H H H H H C C C C C H H H H H H X H C C H H C H H C H H H H C C C H H H H X H H X CO2H (c) Enantiomers Figure 4.7 A-C H C CH3 C C X H CO2H NH2 NH2 C CH3 H MORE ON GEOMETRIC ISOMERS Cis isomer: has non-hydrogen atoms attached to double bonded carbons on the same side of the double bond. Trans isomer has these atoms on opposite sides of the double bond . THALIDOMIDE: --CONTAINS BOTH LEFT- AND RIGHTHANDED ISOMERS IN EQUAL AMOUNTS. --THE (R) ENANTIOMER IS EFFECTIVE AGAINST MORNING SICKNESS. THE (S) CAUSES BIRTH DEFECTS. --THE ENANTIOMERS CAN INTERCONVERT Enantiomers : L-Dopa is a drug used to treat Parkinson’s…the drug’s enantiomer has no effect on patients. Figure 4.8 L-Dopa D-Dopa (effective against Parkinson’s disease) (biologically inactive) FUNCTIONAL GROUPS Functional groups are the parts of molecules involved in chemical reactions They are the chemically reactive groups of atoms within an organic molecule Give organic molecules distinctive chemical properties Figure 4.9 Estradiol OH CH3 HO Female lion OH CH3 CH3 O Male lion Testosterone Six functional groups are important in the chemistry of life Hydroxyl Carbonyl Carboxyl Amino Sulfhydryl Phosphate Study and Learn Figure 4.10 in your text Some important functional groups of organic compounds HYDROXYL CARBONYL O OH (may be written HO STRUCTURE Figure 4.10 CARBOXYL C C OH ) A hydrogen atom is bonds to an oxygen atom, which in turn is bonded to the carbon skeleton of the organic molecule. (Do not confuse this functional group with the hydroxide – ion, OH .) O The carbonyl group ( CO) consists of a carbon atom joined to an oxygen atom by a double bond. When an oxygen atom is double-bonded to a carbon atom that is also bonded to a hydroxyl group, the entire assembly of atoms is called a carboxyl group (—COOH). SOME IMPORTANT FUNCTIONAL GROUPS OF ORGANIC COMPOUNDS Ketones if the carbonyl group NAME OF COMPOUNDS Alcohols (their specific names usually end in -ol) EXAMPLE H H H C C H H is within a carbon skeleton Aldehydes if the carbonyl group is at the end of the carbon skeleton H OH Ethanol, the alcohol present in alcoholic beverages H Figure 4.10 H H C H C H H Acetone, the simplest ketone H acids O C H Carboxylic acids, or organic H H C C H H O C Propanal, an aldehyde H C H O C OH Acetic acid, which gives vinegar its sour taste Some important functional groups of organic compounds AMINO SULFHYDRYL H N H The amino group (—NH2) consists of a nitrogen atom Figure 4.10 bonded to two hydrogen atoms and to the carbon skeleton. PHOSPHATE O SH (may be written HS ) O P OH OH The sulfhydryl group: a sulfur atom bonded to a hydrogen; resembles a hydroxyl group in shape. In a phosphate group, a phosphorus atom is bonded to four oxygen atoms; one oxygen is bonded to the carbon skeleton; two oxygens carry negative charges; abbreviated P .