* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
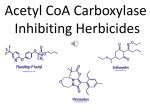
Download Lipid Biosynthesis Inhibitors - Plant and Soil Sciences eLibrary
Basal metabolic rate wikipedia , lookup
Enzyme inhibitor wikipedia , lookup
Peptide synthesis wikipedia , lookup
Plant nutrition wikipedia , lookup
Lipid signaling wikipedia , lookup
Plant breeding wikipedia , lookup
Citric acid cycle wikipedia , lookup
Glyceroneogenesis wikipedia , lookup
Butyric acid wikipedia , lookup
Biochemistry wikipedia , lookup
Specialized pro-resolving mediators wikipedia , lookup
Amino acid synthesis wikipedia , lookup
Biosynthesis wikipedia , lookup
Weed Science Society of America Lipid Biosynthesis Inhibitors Author(s): John W. Gronwald Source: Weed Science, Vol. 39, No. 3 (Jul. - Sep., 1991), pp. 435-449 Published by: Weed Science Society of America and Allen Press Stable URL: http://www.jstor.org/stable/4044977 . Accessed: 01/04/2013 14:36 Your use of the JSTOR archive indicates your acceptance of the Terms & Conditions of Use, available at . http://www.jstor.org/page/info/about/policies/terms.jsp . JSTOR is a not-for-profit service that helps scholars, researchers, and students discover, use, and build upon a wide range of content in a trusted digital archive. We use information technology and tools to increase productivity and facilitate new forms of scholarship. For more information about JSTOR, please contact [email protected]. . Weed Science Society of America and Allen Press are collaborating with JSTOR to digitize, preserve and extend access to Weed Science. http://www.jstor.org This content downloaded from 199.7.208.94 on Mon, 1 Apr 2013 14:36:23 PM All use subject to JSTOR Terms and Conditions Weed Science, 1991. Volume 39:435-449 Lipid BiosynthesisInhibitors1 JOHN W. GRONWALD2 Abstract. Five classes of herbicides (carbamothioates, chloroacetamides, substituted pyridazinones, cyclohexanediones, and aryloxyphenoxypropionic acids) have been reported to inhibit lipid biosynthesis in higher plants. Carbamothioates impair the synthesis of surface lipids (waxes, cutin, suberin). These effects have been attributed to the ability of this herbicide class to inhibit one or more acyl-CoA elongases. Though as yet poorly characterized, these enzymes are associated with the endoplasmic reticulum and catalyze the condensation of malonyl-CoA with fatty acid acyl-CoA substrates to form very longchain fatty acids used in the synthesis of surface lipids. There is contradictory evidence regarding the effects of chloroacetamide herbicides on de novo fatty acid biosynthesis. Selected substituted pyridazinones decrease the degree of unsaturation of plastidic galactolipids. This effect is attributed to the ability of selected members of this herbicide class to inhibit fatty acid desaturases which are thought to be located in the chloroplast envelope. Aryloxyphenoxypropionic acid and cyclohexanedione herbicides inhibit de novo fatty acid biosynthesis in grasses. The target site for these herbicide classes is the enzyme acetyl-CoA carboxylase which is found in the stroma of plastids. In most cases, selectivity between grasses and dicots is expressed at this site. Aryloxyphenoxypropionic acids and cyclohexanediones are reversible, linear, noncompetitive inhibitors of acetyl-CoA carboxylase from grasses. Both classes are also mutually exclusive inhibitors of grass acetyl-CoA carboxylase which suggests that they bind at a common domain on the enzyme. Nomenclature: Acetyl-coenzyme A carboxylase (EC 6.4.1.2). Additional index words. Fatty acid, carbamothioate, chloroacetamide, substituted pyridazinone, cyclohexanedione, aryloxyphenoxypropionate, elongase, desaturase, acetylCoA carboxylase. INTRODUCTION During the past 12 yr, there have been several reviews of lReceived for publicationJuly 30, 1990, and in revised form January11, 1991. Publ. of U.S. Dep. Agric., Agric. Res. Serv. and Minnesota Agric. Exp. St. Paper No. 18,462, Sci. J. Ser., MinnesotaAgric. Exp. Stn., St. Paul, MN. 2PIantPhysiol., Plant Sci. Res. Unit, Agric. Res. Serv., U.S. Dep. Agric. and Assoc. Prof., Dep. Agron. and Plant Genet., Univ. Minnesota, St. Paul, MN 55108. 3Abbreviations: ACCase, acetyl-CoA carboxylase; ATP, adenosine triphosphate;BMS, Black Mexican Sweetcorn; CoA, coenzyme A; DGDG, digalactosyldiacylglycerol; MGDG, monogalactosyldiacylglycerol;Mr relative molecular mass; PG, !Phosphatidylglycerol;VLCFA, very long-chain fatty acids; 16:1(t), trans-A -hexadecenoic acid. the effects of herbicides on various aspects of lipid metabolism in plants (29, 39, 54, 60, 109). Two recent reviews are those of Harwood et al. (54) and Hoppe (60). In this review, five classes of herbicides that have been reportedto interfere with certain aspects of lipid (fatty acid) metabolism will be discussed. These classes are: a) carbamothioates, b) chloroacetamides, c) substituted pyridazinones, d) cyclohexanediones, and e) aryloxyphenoxypropionic acids. Carbamothioates, chloroacetamides, and substitutedpyridazinones are older classes of chemistry that have been reportedto inhibit multiple target sites, including sites other than those involved in lipid biosynthesis (29, 39, 54, 60, 80, 115, 123, 145). For these herbicide classes, the literatureconcerningtheir effects on lipid biosynthesis will be briefly reviewed with an emphasis on recent reports. The major focus of this review will concern the effects of two relatively new herbicide classes, the cyclohexanediones and aryloxyphenoxypropionicacids, on lipid biosynthesis. Within the past 4 yr, considerable advances have been made in elucidating the mechanism of action of these two herbicide classes which are potent inhibitors of de novo fatty acid biosynthesis in grasses (14, 15, 54, 60, 72, 73, 106, 107, 113, 135). A discussion of the details of fatty acid biosynthesis in higher plants will not be provided in this review. The reader is referredto recent reviews by Stumpf (129) and Harwood (50, 51). CARBAMOTHIOATES Representatives of the carbamothioates are shown in Figure 1. These herbicides are applied preplant incorporated and provide selective control of annual grasses and some annualbroadleafweeds duringgerminationand early seedling growth. Symptoms of herbicidal activity in grasses include stunting of early seedling growth and interference with the emergence and unfolding of leaves from the coleoptile (3, 39, 145). The primary site of action of the carbamothioateshas not been identified. The literaturesuggests that the two metabolic processes most sensitive to inhibition by carbamothioatesare gibberellin and lipid biosynthesis (39). Inhibitory effects of carbamothioates on gibberellin biosynthesis have been describedelsewhere (143, 144, 150) and will not be discussed in this review. There is considerable evidence that carbamothioates inhibit the synthesis of surface lipids such as waxes, cutin, and suberin(3, 7, 29, 39, 44, 53, 54, 75, 109, 121, 141, 145, 146, 147). This effect has been attributedto the ability of carbamothioatesto inhibit the biosynthesis of very long-chain fatty acids (VLCFA)3 (7, 39, 53, 54, 75, 121, 145), which are defined as fatty acids of chain length greater than eighteen 435 This content downloaded from 199.7.208.94 on Mon, 1 Apr 2013 14:36:23 PM All use subject to JSTOR Terms and Conditions GRONWALD: LIPID BIOSYNTHESIS NHIBITORS CH3 CH3CH2CH2 ~CH 0 ? N-C -S-CH2 -CH3 CH3CH2CH2 CH3 C CH3 EPTC CH3 OH CH3 CH3 CH3 0 C CH 3 I ,N-C CH -S-CH2 3.5 / CH I I Cl CII -C= Diallate CI 0 N-C = C -S-CH2-C I cI C- I Cl %-3.1 UPPER n\ Triallate Figure 1. Chemical structures of selected carbamothioates. Z 2.9 _X carbons (50). VLCFA are components of surface lipids and they also serve as precursors for such surface lipid components as alkanes, aldehydes, alcohols (primary and secondary), and ketones (74). The first report that carbamothioatesinterfered with the synthesis of surface lipids was that of Gentner (44) who found that EPTC4 (S-ethyl dipropylcarbamothioate)inhibited wax formation on developing leaves of cabbage (Brassica oleracea L.). Since then, the inhibitory effects of carbamothioates on surface lipid biosynthesis have been corroboratedby others. Wilkinson and Hardcastle(147) found that EPTC reduced cuticle thickness on the upper and lower surfaces of sicklepod [Cassia obtusifolia (L.) #5 CASOB] leaflets. The inhibitory effect of increasing EPTC concentration on cuticle thickness in sicklepod is shown in Figure 2. Other studies (75, 121) have demonstrated the inhibitory effect of carbamothioateson the formation of cuticular and epicuticular wax on pea (Pisum sativum L.) leaves. EPTC preferentiallyinhibited the incorporationof [14C]acetateinto surface lipids as opposed to internal lipids in pea (75). Carbamothioateswere also selective inhibitorsof [14C]acetate incorporationinto VLCFA in germinatingpea seedlings (53). In additionto being utilized in the synthesis of epicuticular wax and cutin, VLCFA are also used in the synthesis of suberin found on stem, root, and wound surfaces. If carbamothioatesact as general inhibitors of VLCFA synthesis, they should also inhibit the synthesis of suberin. To test this hypothesis, Bolton and Harwood (7) examined the effect of carbamothioateson the synthesis of suberin in excised potato (Solanum tuberosum L.) discs during aging. This tissue was utilized because it exhibits rapid rates of suberin synthesis (6). EPTC, diallate [S-(2,3-dichloro-2propenyl)bis(I-methylethyl)carbamothioate],and triallate [S- 4Mention of a trademark, vendor, or proprietary product does not constitute a guaranteeor warrantyof the productby the U.S. Dep. Agric. or the Univ. Minnesota, and does not imply its approval to the exclusion of other products or vendors that may also be suitable. 5Letters following this symbol are a WSSA-approved computer code from Composite List of Weeds, Revised 1989. Available from WSSA, 309 West Clark Street, Champaign, IL 61820. 436 UU2.4 / W2.2 LOWER 2.0 0.5 1 2 3 EPTC (kg/ha) 4 Figure 2. Effect of EPTC on cuticle thickness on the upper and lower leaf surface of sicklepod. From Wilkinson and Hardcastle (147). (2,3 ,3-trichloro-2-propenyl) bis( 1-methylethyl)carbamothioate]were selective inhibitorsof fatty acid elongation in excised potato discs. At concentrationsin the range of 10 FM, these herbicides severely reduced the proportion of [14C]acetate incorporated into VLCFA (20:0, 22:0, 24:0) while having little or no effect on the incorporation of radiolabel into palmitic and stearic acid. The evidence is ratherclear that carbamothioatesinterfere with the biosynthesis of surface lipids. It has been proposed that this effect is due to the ability of this herbicide class to inhibit acyl-coenzyme A (CoA)3 elongases (7, 54, 75). These enzymes, found in epidermalcells (1, 4, 83, 84) and cells at wound surfaces (6, 7, 74, 134), are integral membrane proteins associated with the endoplasmic reticulum. AcylCoA elongases have proven to be difficult to isolate and characterize in vitro and only recently has information regardingtheir propertiesbecome available. Most researchon this enzyme system has been conducted with epidermal preparationsfrom leek (Alliumporrum L.) (1, 4, 83, 84) and aged slices of potato tubers (134). Nicotinamide adenine Volume 39, Issue 3 (July-September) 1991 This content downloaded from 199.7.208.94 on Mon, 1 Apr 2013 14:36:23 PM All use subject to JSTOR Terms and Conditions WEED SCEENCE dinucleotide phosphate (NADPH)-dependent elongation of selected acyl-CoA substrates using malonyl-CoA as the condensing agent has been demonstrated in a microsomal preparationisolated from leek epidermal cells (1, 4, 83, 84). Microsomes from leek containedtwo acyl-elongases: one that elongated stearoyl-CoA or palmitoyl-CoA to arachidoyl-CoA and the other that elongated arachidoyl-CoA to very longchain homologs (1). In a study conducted with potato tuber slices, Walker and Harwood (134) obtained evidence for the presence of three separate chain-specific elongases. After slicing potato tubers, there was a sequentialinductionof acylCoA elongases that catalyzed the synthesis of 20:0, 22:0, and 24:0 fatty acids. Recently, the effects of carbamothioateherbicides on in vitro incorporationof [14C]malonyl-CoAinto VLCFA were examined in a microsomal fraction isolated from germinating pea seeds (54). The microsomal fraction isolated from untreated, germinating pea seeds catalyzed the synthesis of VLCFA (20:0, 22:0), but the microsomal fraction isolated from seeds pretreatedwith 100 FM triallate or diallate was not able to synthesize VLCFA. These results indicated that the herbicides (or metabolites such as sulfoxide derivatives) prevented the synthesis of acyl-CoA elongases or inhibited their activity in vivo (54). The in vitro effects of carbamothioates were also examined. Diallate and triallate inhibited elongation of fatty acids in a microsomal preparation from germinating pea seeds. However, rather high concentrations(100 [iM) were requiredand the effect was not selective for the synthesis of VLCFA. Total fatty acid biosynthesis was also strongly inhibited. Two interpretations were suggested to explain the selective effect of carbamothioates on in vivo synthesis of VLCFA in pea (53, 75, 121) but lack of selective effect in vitro (54). It was suggested that a metabolite of the carbamothioate, such as its sulfoxide derivative, could be generated in vivo and act as a selective and potent inhibitor of acyl-CoA elongases. Alternatively, under in vivo conditions, carbamothioatesmay be rapidly metabolizedor poorly translocated,and hence are only able to exert their inhibitory effect on lipid biosynthesis at the plant surface where VLCFA are being synthesized. There are conflicting reports concerning the effects of carbamothioateson de novo fatty acid biosynthesis. EPITC had no effect on the incorporationof [14C]malonicacid into lipids of sesbania [Sesbania exaltata (Raf.) # (SEBEX)] hypocotyl segments (90). However, EPTC and other carbamothioates inhibited the incorporation of radiolabeled precursors ([14C]acetate, [14C]malonate)into fatty acids in isolated spinach (Spinacia oleracea L.) chloroplasts (148, 149). EPIC also inhibited the incorporationof [14C]acetate into lipids of maize (Zea mays L.) cell suspension cultures (38). As discussed above, high concentrations of carbamothioates inhibited de novo fatty acid biosynthesis in a membranefraction isolated from germinatingpea seeds (54). Two hypotheses have been proposed to explain the inhibitory effects of carbamothioateson lipid biosynthesis. A common assumption of both hypotheses is that the sulfoxide derivatives of carbamothioates, formed in vivo, act as alkylating (carbamoylating) agents. Carbamothioates are metabolizedin vivo by a two-stepprocess.The first step, catalyzedby a cytochromeP-450 or a peroxidase,resultsin the formationof a sulfoxidederivative(77, 112).The second stepinvolvesthe conjugationof the sulfoxidederivativewith glutathione(77, 78, 112). One hypothesisproposes that sulfoxidederivativesof carbamothioates alkylate(carbamoylate)key enzymesinvolvedin fattyacidbiosynthesis(43, 78, 112). There is, however, no evidence to support this hypothesis.The second hypothesisproposesthat the carbamothioate-sulfoxide derivative can alkylate CoA and therebyinterferewith CoA metabolism(43, 78). Alkylation of CoA could depletecells of this cofactorwhich plays an role in the transferof acyl groupsduringfattyacid important biosynthesis.Alternatively,the CoA conjugates of carbamothioatesulfoxides could interferecompetitivelywith reactionsutilizing acetyl-CoAor other CoA intermediates. The sulfoxidederivativeof EPIC can alkylateCoA in vitro (78), but it is not knownwhetherthis conjugateis formedin vivo. It is interestingto note thatxenobiotic-CoAconjugates canbe formedin vivo in certainmammalian tissuesandit has been hypothesizedthat these conjugates could inhibit enzymesusing acetyl-CoAor otheracyl-CoAsas substrates (18). Recently,it has been proposedthat the carbamothioate molecule itself (as opposed to its sulfoxide derivative) inhibitskey enzymesinvolvedin the synthesisof acetyl-CoA. Wlkinson and Oswald (151) reported that EPTC, at submicromolar actedas a reversibleinhibitor concentrations, of threeenzymesthat catalyzethe synthesisof acetyl-CoA: the plastidic and mitochrondrialpyruvate dehydrogenase complexes, and the plastidic acetyl-CoAsynthetase.This hypothesisdiffers from the alkylationhypothesisdescribed above in that the carbamothioate, ratherthan its sulfoxide derivative,is the herbicidallyactive form of the molecule, and the interactionis reversibleas opposedto irreversible. CHLOROACETAMIDES The chloroacetamides are appliedpreemergenceand are used primarily for control of grass weeds (80, 115). Representative structures of this herbicideclass are shownin Figure3. Chloroacetamide herbicideshave many properties in commonwith carbamothioates. Bothherbicideclasses are consideredto be generalgrowthinhibitorsthat impairearly seedlinggrowthof grasses(80, 115). Chloroacetamides and carbamothioatesare also similar in their spectrum of selectivityand the injurysymptomswhich they induce in grasses (80, 115). Furthermore, chloroacetamides, like carbamothioates, inhibitseveralmetabolicprocesses(43, 69, 80, 115) but the primarysite of actionhas not been identified. Chloroacetamides inhibitlipidmetabolism(20, 90, 139, 140) as well as the synthesisof proteins(26, 31, 80, 93, 115), gibberellins(80, 115, 142), andproductsof the phenylpropanoid pathway(lignin, anthocyanin)(97). This review will only discuss the purportedeffects of chloroacetamides on lipid metabolism.For informationregardingthe effects of chloroacetamides on othermetabolicprocesses,the readeris referredto two recentreviews (80, 115). Volume 39, Issue 3 (July-September) 1991 This content downloaded from 199.7.208.94 on Mon, 1 Apr 2013 14:36:23 PM All use subject to JSTOR Terms and Conditions 437 GRONWALD: LIPID BIOSYNTlIESIS INHIBITORS CO -CH2C1 H=C-O2N -CO - CH2CI /\N C/H' /NO-H2I H2C=CH-CH2 CH-CH3 - I CH3 Propachlor CDAA CF3 CO-CH2CI 2 "CH OCH3C CH200H3 C2H5 Alachlor NH N2 0 / ~~ NN o Ci NCH3 / 0 ci SAN 6706 Pyrazon NQ ~CH3 N /,CH3 H N Ci BASF 13-338 SAN 9785 CF3 2 2H5 CO-CH2CI CO-CH2CI AN AN CH -CH2 -OCH3 OH, OH3I Metolachlor /CH3 N g \ CH2-OC2H5 OH3 Acetochlor CO-CH2C CH2- N -N -= 0 CI OH3 Metazachlor Figure 3. Chemical structures of selected chloroacetamides. There are contradictoxyreports regarding the effects of chloroacetamideson lipid biosynthesis. Some reportssuggest that chloroacetamides have little or no effect on lipid metabolism. Neither alachlor [2-chloro-N-(2,6-diethylphenyl)N-(methoxymethyl)acetamide]nor metolachlor [2-chloro-N1(2-ethyl-6-methylphenyl)-N-(2-methoxyinhibited incorporation of methylethyl)acetamide] [14C]acetateor [14C]malonicacid into different lipid classes in excised cotton (Gossypium hirsutum L.) root tips (94). Alachlor (8.2 pM) did not alter total lipid biosynthesis or fatty acid composition of sorghum(Sorghumbicolor L.) roots (138). Furthermore,metolachlor (10 p) had no effect on acetate incorporation into total lipids or individual lipid classes of sorghum roots (158). In conflict with the above, there are reportswhich suggest that chloroacetamides inhibit lipid biosynthesis. Butachlor -2 -chloro-N- (2, 6 -diethyl[(N- (buthoxymethyl) phenyl)acetamide] (20) inhibited the incorporation of [14C]acetateinto lipids of isolated leaf cells of red kidney bean (Phaseolus vulgaris L.), while CDAA [2-chloro-N,N-diinhibited incorporation of 2-,propenylacetamide] [14C]malonateinto lipids in excised hypocotyls of sesbania (90). Incorporation of [14C]acetate into lipids of sorghum protoplasts was strongly inhibited by 1 pM metolachlor (159). In the green alga (Scenedesmus acutus), metazachlor [N-(2,6-dimethylphenyl)-N-(1 -pyrazolyl-methyl)-chloroacetamide] inhibited the incorporation of [14C]acetate into polar lipids (139). In further studies with Scenedesmus acutus, Weisshaer et al. (140) reportedthat treating this alga with 5 FM alachlor or metazachlordecreased linolenic acid content but increased the levels of palmitic and oleic acid. The authors concluded that chloroacetamides inhibit fatty acid biosynthesis in Scenedesmus at some point between the elongation of palmitic acid and the desaturationof oleic acid. They speculated that chloroacetamidesmay inhibit plastidic desaturases. In addition to their effects on de novo fatty acid biosynthesis, chloroacetamidesalso inhibit the synthesis and alter the composition of epicuticularwax on the primaryleaf of developing sorghum seedlings (33, 34). The mechanisms proposed to explain the inhibitoryeffects of chloroacetamideson lipid metabolism are similar to those 438 /C~H - Norflurazon Figure 4. Chemical structures of selected substituted pyridazinones. describedabove to explain the inhibitoryeffects of carbamothioateson lipid metabolism.Chloroacetamides, like have been hypothesizedto interferewith carbamnothioates, fatty acid metabolismeither by alkylatingkey enzymes involvedin fattyacidbiosynthesisor by alkylatingCoA and thereby interferingwith CoA metabolism(43, 68, 69). Jaworski(68) firstproposedthatthe mechanismof actionof chloroacetamides mightrelateto their activityas alkylating agents.He postulatedthatCDAA interferedwith respiration and other metabolicprocesses by alkylatingcertain sulfhydryl-containingenzymes. Later work by Hamm (49) demonstrateda strong correlationbetween the herbicidal activity of chloroacetamidesand their ability to act as alkylatingagents. As of yet, there is no evidence that chloroacetamides alkylatespecificenzymesin vivo. However, it has been shown, both in vivo and in vitro, that can alkylateplant proteins(93). chloroacetamides Since the report by Jaworski (68), there has been speculationthat the diverseeffects of chloroacetamides on metabolismmay be relatedto their abilityto alkylateCoA (43, 97). CoAplaysan important rolein lipidmetabolismand othermetabolicprocessesthat are inhibitedby chloroacetamides.The in vitroformationof a CoA conjugateof alachlor has been reported(79). However,thereis no evidencethat CoA conjugatesof the chloroacetamides are formedin vivo. SUBSTITUTEDPYRIDAZINONES The structuresof representative substitutedpyridazinones areshownin Figure4. Minordifferencesin structure of these herbicidesresult in pronounceddifferencesin target site specificity. Dependingon applicationrate and the plant species to which they are applied,the substitutedpyridazinones shown in Figure4 can inhibit one or more of the following:a) photosynthesis,b) carotenoidbiosynthesis,c) fatty acid desaturation(35, 54, 60, 123, 124, 137). The herbicidal effect of pyrazon [5-amino-4-chloro2-phenyl-3(2H)-pyridazinone] is largelydue to its abilityto inhibitphotosynthetic electrontransport(35, 123). Veryhigh concentrations of pyrazonarerequiredbeforeeffectson fatty acidcompositionor carotenoidbiosynthesisareobserved(35, 123). As indicatedby competitivebinding studies, this Volume 39, Issue 3 (July-September) 1991 This content downloaded from 199.7.208.94 on Mon, 1 Apr 2013 14:36:23 PM All use subject to JSTOR Terms and Conditions WEED SCIENCE herbicide binds to D1, the quinone-binding protein on the reducing side of photosystem 1I (123). However, comparedto triazines and ureas, pyrazon is not a potent inhibitor at this site. The I50 values for inhibition of photosystem II electron transportare 7 pM for pyrazon and 0.14 pM for diuron [N'(3,4-dichlorophenyl)-N,N-dimethylurea](123). Norflurazon [4-chloro-5-(methylamino)-2-(3(trifluoromethyl)phenyl)-3(2H)-pyridazinone]causes bleaching in susceptible species (54, 60, 110, 111). It inhibits carotenoidbiosynthesis which results in the photooxidationof chlorophyll. Current evidence suggests that norflurazon blocks carotenoid biosynthesis by inhibiting a desaturase, located in chloroplastmembranes,that catalyzes the desaturation of phytoene. This hypothesis has been difficult to test because this enzyme is unstable during isolation and hence is not readily amenable to in vitro assay (111). However, a recent study conducted with a chromoplast fraction isolated from daffodil (Narcissus pseudonarcissus L.) flowers lends credence to this hypothesis (92). In this system, norflurazon was found to be a reversible, noncompetitive inhibitor of phytoene desaturase. To varying degrees, the substitutedpyridazinonesalter the fatty acid composition of lipids (29, 54, 60). In particular, they increased the degree of saturation of certain lipids, primarilygalactolipids, by blocking the conversion of linoleic acid to linolenic acid (29, 54, 60, 82, 122, 123, 124, 125, 137). Galactolipids [monogalactosyldiacylglycerol (MGDG)3, digalactosyldiacylglycerol(DGDG)3] make up a large portion of chloroplast membranes, and the fatty acids associated with these lipids exhibit a high degree of unsaturation (70). Compared to other substituted pyridazinones, BASF 13-338 (SAN 9785) [4-chlorois rather se5(dimethylamino)-2-phenyl-3(2H)-pyridazinone] lective in its mode of action. It inhibits desaturationof fatty acids with little or no effect on photosynthesis or carotenoid biosynthesis (29, 35, 54, 60, 123). Research by several investigatorshas shown that this herbicide causes an increase in the 18:2/18:3 ratio found in the galactolipid fraction of plant tissues (10, 71, 81, 98, 101, 122, 125, 152). Data illustratingthis effect on the MGDG fraction of wheat shoots are shown in Table 1. Treatmentwith BASF 13-338 caused a pronounced decrease in the level of linolenic acid with a concomitant increase in the level of linoleic acid. The data in Table 1 also show that pyrazon has little effect on the degree of unsaturation of the MGDG fraction. As stated above, pyrazon acts primarily as an inhibitor of photosynthesis (35, 54, 60, 123). It is not surprisingthat BASF 13-338, being a relatively selective inhibitorof fatty acid desaturationin plastids, is not a potent herbicide. Treatment with this herbicide causes minor changes in chloroplast ultrastructure which are attributedto the decrease in linolenate content of plastidic membranes (25, 81). SAN 6706 [4-chloro-5-(dimethylamino)-2(a,a,a-trifluorom-tolyl)-3(2H)-pyridazinone] is a substituted pyridazinone that inhibits both carotenoid biosynthesis and fatty acid desaturation (35, 54, 60, 71, 123). In some species, SAN 6706 is metabolized to norflurazon(128), which explains its Table 1. Effect of substituted pyridazinones on fatty acid composition of MGDG of wheat shoots. Herbicide (0.1 mM) C16:0 Control Pyrazon BASF 13-338 (SAN 9785) Fatty acid composition C18:0 C18:2 C18:1 15.0 17.2 3.3 5.3 21.2 5.0 % by weight 4.3 19.0 21.1 6.1 5.6 53.9 C18:3 58.9 50.2 14.1 From St. John (122). inhibitoryeffect on carotenoidbiosynthesis.At high concentrations,SAN 6706 inhibits the desaturationof 18:2 in galactolipids(25, 71). However,at lower concentrations, it appearsto be a selectiveinhibitorof the desaturation of 16:0 to fonn trans-A3-hexadecenoic acid [16:1(t)]3in the sn-2 position of phosphatidylglycerol, the major phospholipid found in plastids(25, 71). The effects of BASF 13-338 and SAN 6706 on the desaturation of fattyacidshavebeenattributed to theirability to inhibitdesaturasesthat catalyzethese reactions(29, 54, 60). These enzymes,which utilize complexlipids [MGDG, DGDG, phosphatidylglycerol(PG)3] as substrates, are thoughtto be locatedin the chloroplastenvelope(70). It has been hypothesizedthat the chloroplastenvelope contains multipledesaturases(11, 25, 82) andthatthe selectiveeffect of certainsubstitutedpyridazinones is due to theirabilityto selectivelyinhibitone or moreof theseenzymes(29, 54, 60). The selectiveeffectof BASF 13-338on the 18:2/18:3ratioof MGDG(Table1) has been attributed to its abilityto inhibit the A-15 desaturasethat catalyzesthe desaturation bf 18:2 bound to MGDG (25, 29, 54, 60, 82, 98, 152). Likewise, it has been proposedthat SAN 6706 (at low concentrations)is a specific inhibitor of the desaturasecatalyzing the conversion of 16:0 to 16:1(t) at the sn-2 position of phosphatidylglycerol (71). Testing the hypothesis that selected substituted pyridazinones inhibit fatty acid desaturaseshas been difficult because these enzymes have proven to be refractory to in vitro characterization. However, Nornan et al. (102) recently reportedthat a chloroplastmembranefractionisolatedfrom soybeanleavescontaineda desaturase(s) capableof desaturating 18:2to 18:3in eitherthe sn-i or sn-2 positionof MGDG. BASF 13-338 inhibitedthis reaction. ARYLOXYPHENOXYPROPIONIC ACIDS AND CYCLOHEXANEDIONES Introduction.The aryloxyphenoxypropionates andcyclohexanedionesare two relativelynew herbicideclasses that are used for postemergencecontrol of annual and perennial grassesin certainbroadleafcrops (30, 54, 60). Becauseof their selectivity,these two herbicideclasses are sometimes referredto as graminicides. Representative chemistriesof the two classes are shown in Figures5 and 6. Volume 39, Issue 3 (July-September) 1991 This content downloaded from 199.7.208.94 on Mon, 1 Apr 2013 14:36:23 PM All use subject to JSTOR Terms and Conditions 439 GRONWALD: LIPID BIOSYNTHESIS IHITORS CI Table2. Effect of haloxyfop (1 pM on precursoruptakeand incorporationinto radicle tips of corn and soybeana. N CloaN; e ?O-CHCOOC2H5 0 CF3 CH3 / e 0 -CHCOO(CH2),CH, CH, Incubation period (h) 0 Fluazitop-butyl Quizalofop-ethyl 4 8 % of control Cl / \ / O-CHCOOCH3 CH, CF3 / / ~~~~~- Diclofop-methyl O -CHCOOCH3 CH, Haloxyfop-methyl N C O-CHCOOC2H5 CH3 CI Fenoxaprop-ethyl Figure 5. Chemical structures of selected aryloxyphenoxypropionates. Both herbicide classes are foliar applied. They are readily absorbedand translocatedto meristematicregions where they exert their herbicidalactivity imgrasses (8, 30, 46, 54, 58, 60, 64, 66, 86, 87, 130). The aryloxyphenoxypropionatesare applied as esters which are rapidly converted to acids via carboxylesteraseactivity upon entering the leaf (12, 30, 57). The acid form of the herbicide, which is consideredto be the active form, is translocatedto meristematicregions (12, 30, 57). In addition to having a similar spectrumof selectivity, the aryloxyphenoxypropionic acids and the cyclohexanediones cause similar injury symptoms in grasses. Initially, treated plants exhibit chlorosis in developing leaves and a cessation of growth (2, 30, 85, 86, 87). Within a few days, necrosis of the shoot apex and meristematicregions of leaves and roots is apparent (2, 8, 30, 58, 65, 130). Most evidence suggests that the selective action of both the aryloxyphenoxypropionicacids and the cyclohexanediones is not due to differentialabsorptionor translocation(30, 54, 60). Differential metabolism contributesto the selectivity of the aryloxyphenoxypropionicacid diclofop {(?)-2-[4-(2,4dichlorophenoxy)phenoxy]propanoicacid). Wheat (Triticum aestivum L.) is tolerant to diclofop because it can metabolize the herbicide (28, 118). However, for most grasses, the selectivity of the cyclohexanediones and aryloxyphenoxypropionic acids cannot be attributedto differential absorption, translocation, or metabolism which suggests that selectivity residues at the site of action (12, 30, 54, 60). Mechanism of action. The fact that both the aryloxyphenoxypropionic acids and cyclohexanediones cause the same injury symptoms in grasses suggested that they may have a common site of action. Much of the early work regardingthe mechanism of action of the aryloxyphenoxypropionicacids was conducted by Hoppe and co-workers (58, 59, 61, 62). In 1981, Hoppe (58) demonstratedthat lipid biosynthesis in corn (Zea mays L.) radicles was impaired by diclofop. This herbicide strongly inhibited the incorporationof [14C]acetate into fatty acids while having little or no effect on the 440 Corn [14C]Leucine: Uptake Incorporation 93 95 94 97 109 97 [14C]Uracil: Uptake Incorporation 110 95 117 124 95 105 103 109 99 116 103 94 98 106 99 79 90 95 100 106 [14C]Thymine: Uptake Incorporation [14C]Acetate: Uptake Incorporation Soybean [14C]Acetate: Uptake Incorporation 85* 42* 99 111 *Indicates value significantly different from control using the t-test (0.05). aRadicle tips (0.5 cm) were incubated in a buffer containing 1 Jim haloxyfop for the period indicated, then transferredto a solution containing the radiolabeled precursors. Uptake and incorporation of precursor were measured after 30 win. From Stoltenberg (126). incorporation of precursorsinto nucleicacids or proteins.In subsequentstudies,Hoppeand co-workers(59, 61, 62) and others (14, 15, 23, 72, 114, 126, 135) have shown that the aryloxyphenoxypropionic acids are potent inhibitors of [14C]acetateincorporationinto fatty acids of grasses while having little or no effect on [14C]acetateincorporationinto fatty acids of dicots. Data from Stoltenberg (126) illustrates the selective effect of the aryloxyphenoxypropionic acid {2- [4-[[3-chloro-5-(trifluoromethyl)-2haloxyfop pyridinyl]oxy]phenoxy]propanoicacid} on lipid biosynthesis in corn radicles (Table 2). As determined by precursor OH H3 H2CO ~s OCH3 N -O - CH2 -CH3 OH N -O - CH2 -CH, C -CH2 -CH2 -H3 C -CH2 -CH2 -CH3 0 a CH ~CH2 Sethoxydim Cycloxydim OH I N-O-CH2-CH=CH--CI 11H- I C - CH2 -CH3 CH3 CH3 I I H2C CH 's "-OH2 0 Clethodim Figure 6. Chemical structures of selected cyclohexandiones. Volume 39, Issue 3 (July-September) 1991 This content downloaded from 199.7.208.94 on Mon, 1 Apr 2013 14:36:23 PM All use subject to JSTOR Terms and Conditions WEED SCIENCE 7 120 0 o0 6 o o N ~~~~~PEA \ 80 5 0 0 N E 40 4 C)~~~~~~~~~~~1 \ 220 CORN 0 (n 0 2 10-7 10-6 10-5 10-4 10-3 Herbicide Concentration (M) 0 Figure 7. Effect of haloxyfop (-) and sethoxydim (- -) on ACCase activity measured in chloroplast fractions from com (--) and pea (-0-). From Burton et al. (15). 0.1 incorporation studies, lipid biosynthesis was much more sensitive to haloxyfop than was nucleic acid or protein synthesis. Furthermore,the data indicate that impaired lipid biosynthesis in the presence of haloxyfop was not the result of inhibited [14C]acetateuptake. The inhibition of acetate uptake was much less than the inhibition of acetate incorporationand most likely reflected the inhibition of fatty acid biosynthesis. Early studies conducted with the cyclohexanediones also suggested that this herbicide class interfered with lipid metabolism. Burgstahlerand Lichthenthaler(13) reportedthat { 2-[l -(ethoxyimino)butyl]-5-[2sethoxydim (ethylthio)propyl]-3-hydroxy-2-cyclohexen-1-one} reduced glycolipid and phospholipid content of corn seedlings. The authorsconcluded that sethoxydim inhibited an early stage in lipid metabolism in grasses. Further evidence for this hypothesis was provided by Ishiharaet al. (67) who reported that lipid biosynthesis in corn root tips was more sensitive to sethoxydim than was ribonucleic acid (RNA), deoxyribonucleic acid (DNA), or protein synthesis. Several recent reports have demonstratedthat sethoxydim and other cyclohexanediones are potent and selective inhibitors of [14C]acetate incorporation into lipids of sensitive grass species (14, 15, 42, 63, 73, 106, 114). The above studies clearly showed that both classes of graminicides were selective inhibitors of de novo fatty acid biosynthesis (as measured by [14C]acetateincorporation)in grasses. In order to more clearly define the target site of the two herbicide classes, Burton et al. (15) examined the effect of haloxyfop and sethoxydim on the incorporation of [14C]acetate, [14C]pyruvate,and [14C]malonyl-CoAinto fatty acids in isolated corn chloroplasts. Incorporation of 1.0 10.0 Herbicide Concentration (tM) Figure 8. Effect of selected aryloxyphenoxypropionicacids and cyclohexanediones on ACCase activity isolated from BMS maize cell suspension cultures. Fluazifop (-0-), sethoxydim (-A-), clethodim (-U-), cycloxydim (-A-), haloxyfop (-4-), diclofop, (), quizalofop (-*-). Burton and Gronwald, unpublished results. [14C]acetateand [14C]pyruvatewas inhibited 90% or greater by 10 FM sethoxydim and 1 gM haloxyfop, while incorporationof [14C]malonyl-CoAwas slightly stimulated. On the basis of these results, Burton et al. (15) examined the effects of sethoxydim and haloxyfop on acetyl-CoA carboxylase (ACCase)3 activity in chloroplastfractions isolated from corn and pea. Both sethoxydim and haloxyfop were potent inhibitorsof this enzyme from corn chloroplastsbut had little or no effect on the enzyme from pea chloroplasts (Figure 7). The I50 values for inhibition of corn ACCase by sethoxydim and haloxyfop were 4.7 p.M and 0.5 juM, respectively. Sethoxydim (1 mM) or haloxyfop (0.1 mM) did not inhibit ACCase from pea chloroplasts. In further studies conducted with ACCase isolated from Black Mexican Sweetcorn (BMS)3 suspension cultures, it was shown that there were differences in the relative efficacy of selected aryloxyphenoxypropionic acids and cyclohexanediones as inhibitors of this enzyme (Figure 8). The lowest I50 value was obtained with { (?)-2-[4-[(6-chloro-2-quinoxquizalofop alinyl)oxy]phenoxy]propanoic acid} and the highest with sethoxydim and fluazifop {(?)-2-[4-[[5-trifluoromethyl)2-pyridinyl]oxy]phenoxy]propanoicacid}. Within a period of several months during the latterpart of 1987 and the first half of 1988, there were several reports(14, Volume 39, Issue 3 (July-September) 1991 This content downloaded from 199.7.208.94 on Mon, 1 Apr 2013 14:36:23 PM All use subject to JSTOR Terms and Conditions 441 GRONWALD: LIPID BIOSYNTHESIS INHIBITORS 20 -S-) 3 E 15 - ~~~0 S cE 1o _ 5 0 0.1 1.0 10.0 100.0 [Haloxyfop] pM Figure 9. Effect of the S(-) and R(+) enantiomers of haloxyfop on the activity of ACCase isolated from maize leaves. From Secor et al. (114). 15, 42, 72, 73, 106, 107, 113, 136) describing the aryloxyphenoxypropionicacids and the cyclohexanediones as potent and selective inhibitors of ACCase in grasses. These reports provide compelling evidence that ACCase is the site of action of the aryloxyphenoxypropionicacids and cyclohexanediones and that the basis for selectivity between grasses and dicots resides at this site in most cases. It is not surprising that inhibition of ACCase by aryloxyphenoxypropionic acids and cyclohexanediones is lethal in grasses. These herbicides are translocatedto regions of cell division and elongation (shoot and root apices, intercalary meristems) where there is a high demand for malonyl-CoA to support rapid rates of de novo fatty acid biosynthesis. Inhibition of lipid biosynthesis by these two herbicide classes would account for their disruptiveeffects on plastid ultrastructure(8, 86, 87) and membranepermeability (8), and the eventual necrosis that develops in meristematic regions (2, 46, 66, 130). Lipid biosynthesis would not be the only metabolic process disrupted by ACCase inhibitors. Malonyl-CoA also serves as a key intermediate in the synthesis of cuticular waxes, flavonoids, anthocyanins, stilbenoids, and anthraquinones(100, 129). Futher evidence that ACCase is the site of action of the graminicides is provided by: a) the stereospecificity of the activity of aryloxyphenoxypropionicacids at both the whole plant and ACCase level (27, 30, 45, 62, 113, 114); and b) the expression of graminicidetolerance in selected grasses due to either the presence of a tolerantform of ACCase (48, 91, 105, 114) or overproduction of ACCase (104). The aryloxyphenoxypropionicacids exist in two enantiomeric formnsbecause of the 2-substituted propionic acid moiety of the molecule (30). When applied postemergence, the R(+) enantiomers of diclofop (30), haloxyfop (30, 45), quizalofop (133), or fluazifop (27) are herbicidally active on grasses while the S(-) enantiomershave little or no herbicidal activity. When applied to the soil, the S(-) enantiomers of certain aryloxyphenoxypropionicacids (fluazifop, haloxyfop) exhibit some herbicidal activity, but this may reflect conversion of the S(-) form to the R(+) form by soil-borne microorganisms (27, 45). The R(+) enantiomer of arylox442 yphenoxypropionic acids is also much more active as an inhibitor of grass ACCase compared to the S(-) enantiomer (62, 107, 113, 114, 136). The relative efficacy of the R(+) and S(-) enantiomers of haloxyfop as inhibitors of ACCase isolated from corn leaves (114) is shown in Figure 9. Inhibition of ACCase by the S(-) form was attributedto contaminationof this enantiomerform by a small amount of the R(+) enantiomer (114). A few grasses are tolerantto the aryloxyphenoxypropionic acids and cyclohexanediones. With certain grasses, tolerance is due to their ability to metabolize the herbicide (28, 30, 118). For example, wheat is tolerant to diclofop because it can metabolize the herbicide (28, 30, 118). However, for selected graminicide-tolerantgrasses, tolerance at the whole plant level correlateswith tolerance at the level of ACCase. This is observed in red fescue [Festuca rubra (L.) # FESRU] (114, 127), a diclofop-resistant form of Italian ryegrass (Lolium multiflorumLam. # LOLMU) (48) and graminicidetolerant maize lines selected in tissue culture (47, 91, 105). Red fescue is tolerant to sethoxydim (17, 64, 88, 127), tralkoxydim [2-(1-ethoxyimino)propyl]-3-hydroxy-5mesitycyclohex-2-enone] (114), and haloxyfop (114, 127) while tall fescue [Festuca arundinacea (Schreb.) # FESAR], a member of the same genus, is susceptible to these herbicides. In these two fescue species, the differential tolerance to haloxyfop, sethoxydim, and tralkoxydim expressed at the whole plant level paralleled tolerance at the level of ACCase (114, 127). Approximately 4 yr ago, a diclofop-resistant biotpe of Italian ryegrass was found in an Oregon wheat field that had been treatedwith diclofop for at least seven consecutive years (120). Resistance to diclofop was due to the presence of a tolerantform of ACCase (48). Breeding studies indicatedthat resistance was controlled by a single, partially dominant allele encoding a diclofop-insensitive form of ACCase (5). It is interesting to note that the diclofop-resistant Italian ryegrassbiotype from Oregon exhibits little or no toleranceto sethoxydim at either the enzyme (ACCase) or whole plant level (48). Maize lines tolerant to sethoxydim and haloxyfop have been selected from BMS nonregenerable(104) and embrogenic, regenerable cell cultures (47, 91, 105). In the BMS system, the ACCase in tolerant cell lines exhibited no differences in sensitivity to inhibition by sethoxydim or haloxyfop (as determined by 150 values). However, the tolerant lines exhibited higher ACCase activity measured in the absence of the herbicides (104). As determined by Westem blots, the selected cell lines had higher levels of a 220 000 relative molecular mass (Mr)3polypeptide, presumably the subunit of ACCase. These results suggested that tolerancewas due to overproductionof ACCase in the variant lines (104). In the regenerablecell culture system, lines were selected for tolerance to sethoxydim or haloxyfop (47, 105). Tolerance to the herbicides was expressed in the regenerated plants (47, 105). Inheritancestudies indicated that tolerance was controlled by a single, partially dominant allele that encoded for a graminicide-tolerantform of ACCase (91, 105). Breeding studies conducted with five regenerated granniVolume 39, Issue 3 (July-September) 1991 This content downloaded from 199.7.208.94 on Mon, 1 Apr 2013 14:36:23 PM All use subject to JSTOR Terms and Conditions WEED SCIENCE cide-tolerant maize lines selected in tissue culture indicated that resistance to sethoxydim and/or haloxyfop at the whole plant level cosegregated with resistance at the level of ACCase (91, 105). The above reportsprovide strong evidence that ACCase is the primary site of action of the graminicides. However, it also has been proposed that the aryloxyphenoxypropionic acids, and in particulardiclofop, exert their herbicidalactivity in grasses by acting as protonophoresthat dissipate the proton gradient across membranes (24, 116, 117, 154). This mechanism has been referred to as the antiauxin hypothesis (24, 117). Recently, Shimabukuro (116) described the protonophoreeffect of diclofop as the biophysical mechanism of action of this herbicide and suggested that it contributesto the herbicidal activity of diclofop. Since this review concems the effects of herbicides on lipid biosynthesis, the biophysical or antiauxin hypothesis will not be discussed. For a discussion regardingthis proposed mechanism of action, the readeris referredto the recent review by Shimabukuro(116). Properties of ACCase. ACCase is a multifunctional, biotinylated protein located in the stroma of plastids. It catalyzes the ATP-dependentcarboxylationof acetyl-CoA to form malonyl-CoA. This reaction is the first committed step in the de novo synthesis of fatty acids and there is speculation that it might be the rate-limiting step in fatty acid biosynthesis (50, 55, 132). The two partialreactions catalyzed by the enzyme are shown below: (1) Enzyme-biotin +HCO3- + ATP + ADP *-4 Enzyme-biotin-COj- + Pi (2) Enzyme-biotin-CO- + acetyl-CoA <- Malonyl-CoA + Enzyme-biotin. These two reactions are thought to occur at distinct topological sites on the enzyme with the biotin prosthetic group serving as a mobile carboxyl carrierbetween the two sites (50, 52, 129). The catalytic mechanism is described as a hybrid two-site ping pong (41). The ATP-dependentcarboxylation of biotin occurs at the carboxylation site. The biotin prosthetic group then moves to the carboxyltransferasesite where the carboxyl group is transferred to acetyl-CoA forming malonyl-CoA. The ACCase in higher plants has not been as extensively characterizedas the enzyme from mammals (103, 156, 157) or yeast (95). Higher plant ACCase, like mammalianor yeast ACCase, appears to be a multifunctional protein (50, 52). Mammalian ACCase is a dimer with a subunit Mr of approximately 260 000 (50, 52, 103, 156, 157). There is a lack of agreement regardingthe Mr of the native enzyme in higher plants. Reported Mr values for the native enzyme range from approximately400 000 to 800 000 (21, 36, 37, 40, 50, 52, 96, 99, 129). There is also a lack of agreement regardingthe Mr of the ACCase subunit.Reports by Nikolau and colleagues (99, 100) indicated that maize leaf ACCase had a subunit Mr of approximately62 000. However, other reports suggest that the subunitMr of higher plant ACCase is in the range of 200 000 to 240 000 (9, 16, 21, 36, 37, 40, 50, 52, 56, 96). It may be that the smaller subunit (62 000 Mr) reportedfor plant ACCase reflects proteolysis of the enzyme during isolation. Mammalian ACCase is known to be susceptible to proteolysis during isolation (89, 131) and this also appearsto be the case with the plant enzyme (36, 119). Alternatively, the ACCase subunit of 62 000 Mr reportedin maize leaves may representan isozyme of the enzyme (100, 155). It has been proposed that ACCase isozymes exist in plants and that they may serve to regulate the utilization of malonyl-CoA by different metabolic pathways (100, 129). The regulation of higher plant ACCase has not been extensively characterized.Similar to the mammalianACCase, there is evidence that higher plant ACCase is inhibited by malonyl-CoA (41, 99, 107, 108) and palmitoyl-CoA (99). Malonyl-CoA was reported to act as a noncompetitive inhibitor of ACCase from castor oil seed [Ricinus communis (L.)] (41) and wheat leaves (107, 108). The ACCase from rat liver is activated by CoA (156). CoA activated the ACCase from spinach chloroplasts (76) and castor bean seeds (40), had no effect on ACCase from soybean [Glycine max (L.)] seed (21), and inhibited the enzyme from com leaves (99). Like the ACCase from rat liver (157), plant ACCase is regulated by adenine nucleotides. Adenosine diphosphate (ADP) and adenosine monophosphate (AMP) inhibited the activity of wheat germ ACCase by competing for adenosine triphosphate(ATP)3 (32). In other respects, the regulationof higher plant and mammalianACCase differs. In contrast to mammalian ACCase, higher plant ACCase is not allosterically regulatedby phosphorylationor tricarboxylicacids (21, 22, 40, 52, 96, 107, 129). In addition, there is no evidence that plant ACCase forrns polymers in situ as does the mammalian enzyme (50, 52). Mechanism of inhibition of ACCase. Kinetic analyses revealed that aryloxyphenoxypropionicacids and cyclohexanediones are linear, noncompetitive inhibitors of grass ACCase for all three substrates (MgATP, HCO3-, acetylCoA) of the enzyme (16, 106, 107, 108). Kinetic analyses also indicated that the two herbicide classes are reversible inhibitorsthat exhibit a high affinity for the enzyme (16, 106, 107, 108). Of the two partial reactions catalyzed by ACCase, the carboxyltransferasereaction is most sensitive to inhibition by the aryloxyphenoxypropionicacids and cyclohexanediones (16, 106, 107, 108). This is suggested by the fact that in vitro inhibition of ACCase by the two herbicide classes is most sensitive to the concentrationof acetyl-CoA, as opposed to the other substrates of the enzyme (MgATP, HCO3-) (16, 106, 107, 108). Additional evidence for this hypothesis was provided by Rendina et al. (108) who measuredthe effects of diclofop and clethodim {(E,E)-(?)-2-[l-[[3-chloro-2propenyl)oxy]imino]propyl] -5 -[2-(ethylthio)propyl]-3hydroxy-2-cyclohexen-1-one} on the two partial reactions of wheat ACCase. The partial reaction measured at the carboxyltransferasesite (malonyl-CoA -['4C]acetyl-CoA exchange) was much more sensitive to these herbicides than that measuredat the carboxylationsite (ATP-32Pi exchange). For both the aryloxyphenoxypropionicacids and cyclohexanediones, the family of lines generated by kinetic analyses of the inhibition of ACCase versus the three substratesof the Volume 39, Issue 3 (July-September) 1991 This content downloaded from 199.7.208.94 on Mon, 1 Apr 2013 14:36:23 PM All use subject to JSTOR Terms and Conditions 443 GRONWALD: LIPID BIOSYNTHESIS INHIBITORS Acetate Pyruvate PLASTID :2-M AryloxyphenoxyAcetyl-CoA > '| ACCase propionic Acids Cyclohexanediones Substituted P Syridazinones esatu rase 1:3 Malonyl-CoA - MGDG {FAS 16:0 - ACP .- 16:0 CoA 18:0 - CoA < ?18:0 - ACP - o18:1 - ACP 0 M on Very Long Chain Wax Suberin Cutin FattyAcidsWa,SbrnCui CoA ' ENDOPLASMIC RETICULUM Carbamothioates Figure 10. Simplifiedschematicof fattyacidbiosynthesis in higherplantsillustrating proposedsitesof actionfor thecarbamothioate, substituted pyridazinone, aryloxyphenoxypropionic acid, andcyclohexanedione herbicides.Takenin partfromHarwood(54). Abbreviations: ACCase,acetyl-CoAcarboxylase; ACP, acyl carrierprotein;ACS, acetyl-CoAsynthetase;CoA, coenzymeA; FAS, fatty acid synthase;MGDG,monogalactosyldiacylglycerol; PDC, pyruvate dehydrogenase complex. enzyme are strikingly similar (16, 107, 108). This suggested that the herbicides may bind at a common domain on the enzyme. Multiple inhibition kinetic analyses have been used to test this hypothesis. The results of two separate studies demonstrated that the aryloxyphenoxypropionic acids and cyclohexanediones are mutually exclusive inhibitors of grass ACCase (16, 108). These results may indicate that the two chemistries bind at a common site. However, it is not necessary that two inhibitors bind at a common site in order to be mutually exclusive. For two compounds to be mutually exclusive simply means that the binding of one compound prevents the binding of the other. It is possible that the cyclohexanediones and the aryloxyphenoxypropionatesbind at different sites on ACCase, with the binding of one herbicide class inducing an allosteric change that preventsthe binding of the other. Additional multiple inhibition kinetic analyses conducted with wheat ACCase indicated that both diclofop and clethodim are mutually exclusive for malonyl-CoA but not CoA (108). It was postulated that the aryloxyphenoxypropionic acids and the cyclohexanediones have a common structuralfeature that overlaps with the thioester region of acetyl-CoA and malonyl-CoA. Recently, it has been proposed that the cyclohexanediones may act as stable transition-stateanalogues of the complex 444 formed at the carboxyltransferase site of ACCase (153). Evidencein supportof this is basedsolely on modelingusing moleculargraphics.It is interestingthat the cyclohexanediones (andaryloxyphenoxypropionic acids)have propertiesas inhibitors that would be expected for transition state analogues;i.e., they arereversible,noncompetitive inhibitors that exhibit a high affinityfor a bindingsite. SUMMARY A simplifiedschematicof lipid biosynthesisin higher plants(Figure10) will be used to summarizewhathas been discussed regardingthe effects of the selected herbicide classes on lipid biosynthesis. Carbamothioates.Carbamothioates inhibitthe synthesisof surfacelipids presumablyby inhibitingacyl-CoAelongases which catalyzethe synthesisof VLCFA(7, 54, 75). AcylCoA elongases have not yet been well characterizedbut appearto be associatedwith the endoplasmicreticulumand catalyzethe condensation of malonyl-CoAwith variousacylCoA fattyacidprimers(1, 4, 83, 84). Shownin Figure10 is the inhibition of elongation of stearoyl-CoAby carbamothioates. Evidence suggesting that carbamothioatesinhibit one or more acyl-CoA elongases is largely indirect. Treatmentwith Volume 39, Issue 3 (July-September) 1991 This content downloaded from 199.7.208.94 on Mon, 1 Apr 2013 14:36:23 PM All use subject to JSTOR Terms and Conditions WEED SCIENCE carbamothioatesreduces the amountof surface lipid on plants (7, 53, 75, 141, 145, 147). Furthermore,in vivo studies with [14C]acetatehave indicated that elongation of C:16 and C:18 fatty acids is more sensitive to these herbicides than is their de novo synthesis (7, 75). Recently, in vitro studies indicated that diallate and triallate inhibitedthe synthesis of VLCFA in a microsomal fraction from germinating pea seed (54). However, relatively high concentrations (100 il) of the herbicides were requiredand total fatty acid biosynthesis was also strongly inhibited at these concentrations. It has been proposed that the sulfoxide derivatives of carbamothioates, which are known to be formed in vivo (19, 79), or CoA conjugates of these derivatives, which have been hypothesized to be formed in vivo (43), may inhibit lipid synthesis. It is possible that one of these carbamothioatemetabolites may selectively inhibit acyl-CoA elongases in vivo. Further research is needed to address this question. The effects of carbamothioateson surfacelipids are one of the best documentedeffects of this herbicide class. However, this effect probably makes only a minor contributionto the herbicidal activity of carbamothioates. It is unlikely that impaired synthesis of cutin, wax, and suberinwould be toxic in itself. Chloroacetamides. There is not a clear picture regardingthe effects of the chloroacetamides on lipid biosynthesis. Chloroacetamides,like carbamothioates,inhibit the synthesis of epicuticularwax (33, 34). However, reportsconceming the effects of this herbicide class on de novo fatty acid biosynthesis are contradictory. Recently, Weisshaar et al. (140) suggested that this herbicide class may inhibit plastidic desaturases. Chloroacetamides (like carbamothioates)have been proposed to interfere with lipid metabolism by alkylating CoA or key enzymes involved in lipid metabolism (43, 68, 69). Substituted pyridazinones. Certain representatives of this chemistry appearto inhibit one or more fatty acid desaturases associated with the chloroplast envelope (29, 54, 60). Based on the in vivo effects of BASF 13-338 on fatty acid composition, this herbicide appears to be a rather selective inhibitor of the A-15 desaturaseresponsible for desaturation of 18:2 bound to MGDG (29, 54, 60) (Figure 10). Recently, it was demonstrated in vitro that BASF 13-338 inhibits desaturationof 18:2 bound to MGDG (102). Although not as selective in its mechanism of action as is BASF 13-338, SAN 6706 apparentlyinhibits the desaturaseconverting 16:0 to 16:1(t) in the sn-2 position of phosphatidylglycerol (25, 29, 54, 60, 71). BASF 13-338 and other substituted pyridazinones should prove to be useful tools to learn more about fatty acid desaturasesin plants and their role in plant adaptation to various stresses. While the substituted pyridazinones inhibit fatty acid desaturationto varying degrees, it does not appear that this effect makes a significant contribution to their herbicidal activity. BASF 13-338, the substituted pyridazinone that selectively inhibits fatty acid desaturationwith little or no effect on photosynthesis or carotenoid biosynthesis, has minimal phytotoxicity to plants. The most effective herbicides of the substituted pyridazinones are those that also inhibit photosynthesis and/or carotenoid biosynthesis (123). Aryloxyphenoxypropionic acids/cyclohexanediones. There is strong evidence that aryloxyphenoxypropionicacids and cyclohexanediones inhibit ACCase, a key enzyme that catalyzes the first committed step in de novo biosynthesis of fatty acids (14, 15, 42, 72, 106, 107, 113) (Figure 10). Of the two partialreactionscatalyzed by ACCase, the carboxyltransferase reaction is most susceptible to inhibition by these chemistries(16, 107, 108). Both chemistries appearto bind at a common domain on ACCase (16, 107, 108). The aryloxyphenoxypropionic acids and cyclohexanediones should prove to be useful tools to furtherour understanding about this important enzyme and to provide information about the intriguing difference between grass and dicot ACCase which governs the selectivity of these herbicide classes. LITERATURE CITED 1. Agrawal, V. P. and P. K. Stumpf. 1985. Characterizationand solubilizationof an acyl chain elongation system in microsomes of leek epidermal cells. Arch. Biochem. Biophys. 240:154-165. 2. Asare-Boamab,N. K. and R. A. Fletcher. 1983. Physiological and cytological effects of BAS 9052 OH on corn (Zea mays) seedlings. Weed Sci. 31:49-55. 3. Ashton, F. M. and A. S. Crafts. 1981. Thiocarbamates.Pages 303-327 in Mode of Action of Herbicides. John Wiley and Sons, New York. 4. Bessoule, J-J, R. Lessire, and C. Cassagne. 1989. Partial purification of the acyl-CoA elongase of Allium porrum leaves. Arch. Biochem. Biophys. 268:475-484. 5. Betts, K. J., D. L. Wyse, J. W. Gronwald, and N. J. Ehlke. 1990. Inheritanceof diclofop resistance in Italian ryegrass. Abstr. Weed Sci. Soc. Am. 30:58. 6. Bolton, P. and J. L. Harwood. 1976. Fatty acid synthesis in aged potato slices. Phytochemistry 15:1501-1506. 7. Bolton, P. and J. L. Harwood. 1976. Effect of thiocarbamate herbicides on fatty acid synthesis by potato. Phytochemistry 15: 1507-1509. 8. Brezeanu, A. G., D. G. Davies, and R. H. Shimabukuro. 1976. Ultrastructuraleffects and translocation of methyl-2-(4-(2,4-dichlorophenoxy)phenoxypropanoatein wheat (Triticumaestivum) and wild oat (Avena fatua). Can. J. Bot. 54:2038-2048. 9. Brock, K. and C. G. Kannangara.1976. Propertiesand morphology of barley embryo acetyl CoA carboxylase. CarlsbergRes. Commun. 41:121-129. 10. Brockman, J. A., H. A. Norman, and D. F. Hildebrand. 1990. Effects of temperature,light and a chemical modulatoron linolenate biosynthesis in mutant and wild type Arabidopsis calli. Phytochemistry 29:1447-1453. 11. Browse, J., P. McCourt, and C. Somerville. 1986. A mutant of Arabidopsis deficient in C18:3and C16:3 leaf lipids. Plant Physiol. 81:859-864. 12. Buhler, D. D., B. A. Swisher, and 0. C. Bumside. 1985. Behavior of 14C-haloxyfop-methylin intact plants and cell cultures. Weed Sci. 33:291-299. 13. Burgstahler, R. J. and H. K. Lichtenthaler. 1984. Inhibition by sethoxydim of phospho- and galactolipid accumulation in maize seedlings. Pages 619-622 in P. A. Siegenthalerand W. Eichenberger, eds. Structure, Function and Metabolism of Plant Lipids. Elsevier, Amsterdam. 14. Burton,J. D., J. W. Gronwald,D. A. Somers, J. A. Connelly, B. G. Gengenbach, and D. L. Wyse. 1987. Inhibition of plant acetylcoenzyme A carboxylase by the herbicides sethoxydim and haloxyfop. Biochem. Biophys. Res. Cornmun. 148:1039-1044. 15. Burton, J. D., J. W. Gronwald, D. A. Somers, B. G. Gengenbach, Volume 39, Issue 3 (July-September) 1991 This content downloaded from 199.7.208.94 on Mon, 1 Apr 2013 14:36:23 PM All use subject to JSTOR Terms and Conditions 445 GRONWALD: LIPID BIOSYNTBESIS INHIBITORS 16. 17. 18. 19. 20. 21. 22. 23. 24. 25. 26. 27. 28. 29. 30. 31. 32. 33. 34. 35. 36. 37. 446 and D. L. Wyse. 1989. Inhibition of corn acetyl-CoA carboxylase by cyclohexanedione and aryloxyphenoxypropionateherbicides. Pestic. Biochem. Physiol. 34:76-85. Burton, J. D., J. W. Gronwald, R. A. Keith, D. A. Somers, B. G. Gengenbach, and D. L. Wyse. 1991. Kinetics of inhibition of acetyl-coenzyme A carboxylase by sethoxydim and haloxyfop. Pestic. Biochem. Physiol. 39:100-109. Butler, J.H.B. and A. P. Appleby. 1986. Tolerance of red fescue (Festuca rubra) and bentgrass (Agrostis spp.) to sethoxydim. Weed Sci. 34:457-461. Caldwell, J. 1984. Xenobiotic acyl-coenzymes A: Critical intermediates in the biochemical pharmacology and toxicology of carboxylic acids. Biochem. Soc. Trans. 12:9-11. Carringer,R. D., C. E. Rieck, and L. P. Bush. 1978. Metabolismof EPTC in com (Zea mays). Weed Sci. 26:157-160. Chang, S-S, F. M. Ashton, and D. E. Bayer. 1985. Butachlor influence on selected metabolic processes of plant cells and tissues. J. Plant Growth Regul. 4:1-9. Charles,D. J. and J. H. Cherry. 1986. Purificationand characterization of acetyl-CoA carboxylase from developing soybean seeds. Phytochemistry 25:1067-1071. Charles, D. J., P. M. Hasegawa, and J. H. Cherry. 1986. Characterizationof acetyl-CoA carboxylase in the seed of two soybean genotypes. Phytochemistry 25:55-59. Cho, H-Y, J. M. Widholm, and F. W. Slife. 1986. Effects of haloxyfop on com (Zea mays) and soybean (Glycine max) cell suspension cultures. Weed Sci. 34:496-501. Cobb, A. H. and P. Barnwell. 1989. Anti-auxin activity of graminicides. Brighton Crop. Prot. Conf.-Weeds. 1:183-190. Davies, A. 0. and J. L. Harwood. 1983. Effect of substituted pyridazinones on chloroplast structure and lipid metabolism in greening barley leaves. J. Exp. Bot. 34:1089-1100. Deal, L. M., J. T. Reeves, B. A. Larkins,and F. D. Hess. 1980. Use of an in vitro protein synthesizing system to test the mode of action of chloroacetamides. Weed Sci. 28:334-340. Dicks, J. W., J. W. Slater, and D. W. Bewick. 1985. PP 005-The Renantiomerof fluazifop-butyl.Proc. Br. CropProt. Conf.-Weeds. 1: 271-280. Donald, W. W. and R. H. Shimnabukuro.1980. Selectivity of diclofop-methyl between wheat and wild oat: Growth and herbicide metabolism. Physiol. Plant. 49:459-464. Duke, S. 0. 1985. Effects of herbicides on nonphotosynthetic biosynthetic processes. Pages 99-112 in S. 0. Duke, ed. Weed Physiology. Vol. II. Herbicide Physiology. CRC Press, Boca Raton, FL. Duke, S. 0. and W. H. Kenyon. 1988. Polycyclic alkanoic acids. Pages 71-116 in P. C. Kearney and D. D. Kaufman, eds. Herbicides: Chemistry, Degradation, and Mode of Action. Vol. 3. Marcel-Dekker, New York. Duke, W. B., F. W. Slife, J. B. Hanson, and H. S. Butler. 1975. An investigation of the mechanism of action of propachlor.Weed Sci. 23:142-147. Eastwell, K. C. and P. K. Stumpf. 1983. Regulation of plant acetylCoA carboxylase by adenylatenucleotides. Plant Physiol. 72:50-55. Ebert, E. 1982. The role of waxes in the uptake of metolachlorinto sorghum in relation to the protectantCGA 43089. Weed Res. 22: 305-311. Ebert, E. and K. Ramsteiner. 1984. Influence of metolachlorand the metolachlor protectant CGA 43089 on the biosynthesis of epicuticular waxes on the primary leaves of Sorghum bicolor Moench. Weed Res. 24:383-389. Eder, F. A. 1979. Pyridazinones,their influence on the biosynthesis of carotenoids and the metabolism of lipids in plants (survey of literature). Z. Naturforsch. 34c:1052-1054. Egin-Buhler, B. and J. Ebel. 1983. Improved purification and further characterizationof acetyl-CoA carboxylase from cultured cells of parsley (Petroselinum hortense). Eur. J. Biochem. 133: 335-339. Egin-Buhler,B., R. Loyal, and 3. Ebel. 1980. Comparisonof acetylCoA carboxylases from parsley cell cultures and wheat germ. Arch. Biochem. Biophys. 203:90-100. 38. Ezra, G., J. Gressel, and H. M. Flowers. 1983. Effects of the herbicide EPITCand the protectant DDCA on incorporationand distributionof [2-14C]acetate into major lipid fractions of maize cell suspension cultures. Pestic. Biochem. Physiol. 19:225-234. 39. Fedtke, C. 1982. Biochemistry and Physiology of Herbicide Action. Pages 142-147. Springer-Verlag,New York. 40. Finlayson, S. A. and D. T. Dennis. 1983. Acetyl-coenzyme A carboxylase from the developing endosperm of Ricinus communis. I. Isolation and characterization.Arch. Biochem. Biophys. 225: 576-585. 41. Finlayson, S. A. and D. T. Dennis. 1983. Acetyl-coenzyme A carboxylase from the developing endosperm of Ricinus communis. IH.A two-site kinetic mechanism. Arch. Biochem. Biophys. 225: 586-595. 42. Focke, M. and H. K. Lichtentbaler. 1987. Inhibition of the acetylCoA carboxylase of barley chloroplasts by cycloxydim and sethoxydim. Z. Naturforsch. 42c:1361-1363. 43. Fuerst, E. P. 1987. Understanding the mode of action of the chloroacetamide and thiocarbamateherbicides. Weed Technol. 1: 270-277. 44. Gentner, W. A. 1966. The influence of EPTC on extemal foliage wax deposition. Weeds. 14:27-31. 45. Gerwick, B. C., L. A. Jackson, J. Handly, N. R. Gray, and J. W. Russell. 1988. Preemergence and postemergence activities of the (R) and (S) enantiomers of haloxyfop. Weed Sci. 36:453-456. 46. Gronwald,J. W. 1986. Effect of haloxyfop and haloxyfop-methyl on elongation and respiration of corn (Zea mays) and soybean (Glycine max) roots. Weed Sci. 34:196-202. 47. Gronwald,J. W., W. B. Parker,D. A. Somers, D. L. Wyse, and B. G. Gengenbach. 1989. Selection for tolerance to graminicide herbicides in maize tissue culture. Proc. Brighton Crop Prot. Conf.-Weeds. 3:1217-1224. 48. Gronwald,J. W., C. V. Eberlein, K. J. Betts, K. M. Rosow, N. J. Ehlke, and D. L. Wyse. 1989. Diclofop resistance in a biotype of Italian ryegrass. Plant Physiol. 89:S-115. 49. Hamm, P. C. 1972. Some unique biological activity-structure relationships of the acylated anilides of the alachlor type. Pages 41-64 in A. S. Tahori, ed. Herbicides, Fungicides, Formulation Chemistry,Proc. 2nd Int. Congr. Pestic. Chem. (UPAC). Vol. V. Gordon and Breach, New York. 50. Harwood, J. L. 1988. Fatty acid metabolism. Annu. Rev. Plant Physiol. Plant Mol. Biol. 39:101-138. 51. Harwood, J. 1989. Lipid metabolism in plants. Pages 1-43 in B. V. Conger, ed. Crit. Rev. Plant Sci. Vol. 8. CRC Press, Inc., Boca Raton, FL. 52. Harwood, J. L. 1989. The properties and importance of acetylcoenzyme A carboxylase in plants. Proc. Brighton Crop Prot. Conf.-Weeds. 1:155-162. 53. Harwood, J. L. and P. K. Stumpf. 1971. Fat metabolism in higher plants: Control of fatty acid synthesis in germinating seeds. Arch. Biochem. Biophys. 142:281-291. 54. Harwood, J. L., S. M. Ridley, and K. A. Walker. 1989. Herbicides inhibiting lipid synthesis. Pages 73-96 in A. D. Dodge, ed. Herbicides and Plant Metabolism. Cambridge University Press, New York. 55. Hawke, J. C. and R. M. Leech. 1987. Acetyl-CoA-carboxylase activity in normally developing wheat leaves. Planta 171:489-495. 56. Hellyer, A., H. E. Bambridge,and A. R. Slabas. 1986. Plant acetylCoA carboxylase. Biochem. Soc. Trans. 14:565-568. 57. Hendley, P., J. W. Dicks, T. J. Monaco, S. M. Slyfield, 0. J. Tummon, and J. C. Barrett. 1985. Translocationand metabolism of pyridinyloxyphenoxypropionateherbicides in rhizomatous quackgrass (Agropyron repens). Weed Sci. 33:11-24. 58. Hoppe, H. H. 1981. Effect of diclofop-methyl on protein, nucleic acid, and lipid biosynthesis in tips of radicles from Zea mays L. Z. Pflanzenphysiol. 102:189-197. 59. Hoppe, H. H. 1985. Differential effect of diclofop-methyl on fatty acid biosynthesis in leaves of sensitive and tolerant plant species. Pestic. Biochem. Physiol. 23:297-308. Volume 39, Issue 3 (July-September) 1991 This content downloaded from 199.7.208.94 on Mon, 1 Apr 2013 14:36:23 PM All use subject to JSTOR Terms and Conditions WEED SCIENCE 60. Hoppe, H. H. 1989. Fatty acid biosynthesis - a target site of herbicide action. Pages 65-83 in P. B6ger and G. Sandmann,eds. Target Sites of Herbicide Action. CRC Press, Inc., Boca Raton, FL. 61. Hoppe, H. H. and H. Zacher. 1982. Inhibition of fatty acid biosynthesis in tips of radicles from Zea mnaysby diclofop-methyl. Z. Pflanzenphysiol. 106:287-298. 62. Hoppe, H. H. and H. Zacher. 1985. Inhibition of fatty acid biosynthesis in isolated bean and maize chloroplasts by herbicidal phenoxy-phenoxypropionicacid derivatives and structurallyrelated compounds. Pestic. Biochem. Physiol. 24:298-305. 63. Hosaka, H. and M. Takagi. 1987. Biochemical effects of sethoxydim in excised root tips of corn (Zea mays). Weed Sci. 35: 612-618. 64. Hosaka, H., H. Inaba, and H. Ishikawa. 1984. Response of monocotyledons to BAS 9052 OH. Weed Sci. 32:28-32. 65. Hosaka, H., H. Inaba, A. Satoh, and H. Ishikawa. 1984. Morphological and histological effects of sethoxydim on com (Zea mays) seedlings. Weed Sci. 32:711-721. 66. Ikai, T., K. Suzuki, K. Hattori,H. Igarashi,and N. Uchiyama. 1985. The site of action of quizalofop-ethyl, NCI-96683. Br. Crop Prot. Conf.-Weeds. 1:163-169. 67. Ishihara, K., H. Hosaka, M. Kubota, H. Kamimura,N. Takakusa, and Y. Yasuda. 1987. Effects of sethoxydim on the metabolism of excised root tips of com. Pages 187-190 in R. Greenhalghand T. R. Roberts, eds. Pesticide Chemistry and Technology. Blackwell Scientific, Palo Alto, CA. 68. Jaworski, E. G. 1956. Biochemical action of CDAA, a new herbicide. Science 123:847-848. 69. Jaworski, E. G. 1969. Analysis of the mode of action of herbicidal a-chloroacetamides J. Agric. Food Chem. 17:165-170. 70. Joyard, J. and R. Douce. 1987. Galactolipid synthesis. Pages 215-274 in P. K. Stumpf and E. E. Conn, eds. Biochemistry of Plants. Vol. 9. Lipids: Structure and Function. Academic Press, New York. 71. Khan, M-U, N. W. Lem, K. R. Chandorkar,and J. P. Williams. 1979. Effects of substituted pyridazinones (San 6706, San 9774, San 9785) on glycerolipids and their associated fatty acids in the leaves of Vicia faba and Hordeum vulgare. Plant Physiol. 64: 300-305. 72. Kobek, K., M. Focke, and H. K. Lichtenthaler. 1988. Fatty-acid biosynthesis and acetyl-CoA carboxylase as a target of diclofop, fenoxaprop and other aryloxy-phenoxy-propionicacid herbicides. Z. Naturforsch. 43c:47-54. 73. Kobek, K., M. Focke, H. K. Lichtenthaler, G. Retzlaff, and B. Wurzer. 1988. Inhibition of fatty acid biosynthesis in isolated chloroplasts by cycloxydim and other cyclohexane-1,3-diones. Physiol. Plant. 72:492-498. 74. Kolattukudy,P.E. 1980. Cutin, suberin, and waxes. Pages 571-645 in P. K. Stumpf, ed. The Biochemistry of Plants. Vol. 4. Lipids: Structure and Function. Academic Press, New York. 75. Kolattukudy,P. E. and L. Brown. 1974. Inhibitionof cuticularlipid biosynthesis in Pisum sativum by thiocarbamates.Plant Physiol. 53: 903-906. 76. Laing, W. A. and P. G. Roughan. 1982. Activation of spinach chloroplast acetyl-coenzyme A carboxylase by coenzyme A. FEBS Lett. 144:341-344. 77. Lamoureux, G. L. and D. S. Frear. 1979. Pesticide metabolism in higher plants: In vitro enzyme studies. Pages 77-128 in G. D. Paulson, D. S. Frear,and E. P. Marks, eds. Xenobiotic Metabolism, In Vitro Methods. Am. Chem. Soc. Symp. Ser. 97. Am. Chem. Soc., Washington, DC. 78. Lay, M-M and J. E. Casida. 1976. Dichloroacetamide antidotes enhance thiocarbamatesulfoxide detoxification by elevating corn root glutathione content and glutathione S-transferase activity. Pestic. Biochem. Physiol. 6:442-456. 79. Leavitt, J.R.C. and D. Penner. 1979. In vitro conjugation of glutathione and other thiols with acetanilide herbicides and EPTC sulfoxide and the action of the herbicide antidoteR-25788. J. Agric. Food Chem. 27:533-536. 80. LeBaron, H. M., J. E. McFarland, and B. J. Simoneaux. 1988. Metolachlor.Pages 335-381 in P. C. Keamey and D. D. Kaufman, eds. Herbicides: Chemistry, Degradation and Mode of Action. Marcel-Dekker, New York. 81. Leech, R. M., C. A. Walton, and N. R. Baker. 1985. Some effects of 4-chloro-5-(dimethylamino)-2-phenyl-3(2H)-pyridazinone(San 9785) on the development of chloroplast thylakoid membranes in Hordeum vulgare L. Planta 165:277-283. 82. Lem, N. W. and J. P. Williams. 1981. Desaturationof fatty acids associated with monogalactosyl diacylglycerol: The effects of San 6706 and San 9785. Plant Physiol. 68:944-949. 83. Lessire, R., J-J. Bessoule, and C. Cassagne. 1985. Solubilizationof C18-CoA and C20-CoA elongases from Allium porrum L. epidermal cell microsomes. FEBS Lett. 187:314-320. 84. Lessire, R., J-J. Bessoule, and C. Cassagne. 1989. Involvement of a [3-ketoacyl-CoAintermediatein acyl-CoA elongation by an acylCoA elongase purified from leek epidermal cells. Biochim. Biophys. Acta 1006:35-40. 85. Lichtenthaler,H. K. 1984. Chloroplastbiogenesis, its inhibitionand modification by new herbicide compounds. Z. Naturforsch. 39c: 492-499. 86. Lichtenthaler,H. K. and D. Meier. 1984. Inhibitionby sethoxydim of chloroplast biogenesis, development and replication in barley seedlings. Z. Naturforsch. 39c:115-122. 87. Lichtenthaler,H. K., K. Kobek, and K. Ishii. 1987. Inhibition by sethoxydim of pigment accumulationand fatty acid biosynthesis in chloroplasts of Avena seedlings. Z. Naturforsch. 42c:1275-1279. 88. Lichtenthaler,H. K., K. Kobek, and M. Focke. 1989. Differences in sensitivity and tolerance of monocotyledonous and dicotyledonous plants towards inhibitors of acetyl-coenzyme A carboxylase. Proc. Brighton Crop Prot. Conf.-Weeds. 1:173-182. 89. Mackall, J. C. and M. D. Lane. 1977. Changes in mammary-gland acetyl-coenzyme A carboxylase associated with lactogenic differentiation. Biochem. J. 162:635-642. 90. Mann,J. D. and M. Pu. 1968. Inhibitionof lipid synthesis by certain herbicides. Weed Sci. 16:197-198. 91. Marshall,L. C. 1990. Characterizationof maize genotypes tolerant to herbicides that inhibit acetyl-CoA carboxylase. Ph.D. Thesis. Univ. Minnesota. 91 pp. 92. Mayer, M. P., D. L. Bartlett,P. Beyer, and H. Kleinig. 1989. The in vitro mode of action of bleaching herbicides on the desaturationof 15-cis-phytoene and cis-8-carotene in isolated daffodil chromoplasts. Pestic. Biochem. Physiol. 34:111-117. 93. McFarland,J. E. 1986. Alkylation:A possible mode of action of the chloroacetanilide herbicides. Ph.D. Thesis. Purdue Univ. 96 pp. 94. Mellis, J. M., P. Pillai, D. E. Davis, and B. Truelove. 1982. Metolachlor and alachlor effects on membrane permeability and lipid synthesis. Weed Sci. 30:399-404. 95. Mishina,M., T. Kamiiyo, A. Tanaka,S. Fukui, and S. Numa. 1976. Acetyl-coenzyme-A carboxylase of Candida lipolytica. 1. Purification and properties of the enzyme. Eur. J. Biochem. 71:295-300. 96. Mohan, S. B. and R.G.O. Kekwick. 1980. Acetyl-coenzyme A carboxylasefrom Avocado (Persea americana) plastids and spinach (Spinacia oleracea) chloroplasts. Biochem. J. 187:667-676. 97. Molin, W. T., E. J. Anderson, and C. A. Porter. 1986. Effects of alachlor on anthocyanin and lignin synthesis in etiolated sorghum [Sorghum bicolor (L.) Moench.] mesocotyls. Pestic. Biochem. Physiol. 25:105-111. 98. Murphy,D. J., J. L. Harwood, K. A. Lee, F. Roberto, P. K. Stumpf, and J. B. St. John. 1985. Differential responses of a range of photosynthetic tissues to a substituted pyridazinone, SANDOZ 9785. Specific effects on fatty acid desaturation.Phytochemistry24: 1923-1929. 99. Nikolau, B. J. and J. C. Hawke. 1984. Purification and characterization of maize leaf acetyl-coenzyme A carboxylase. Arch. Biochem. Biophys. 228:86-96. 100. Nikolau, B. J., E. S. Wurtele, and P. K. Stumpf. 1984. Tissue distribution of acetyl-coenzyme A carboxylase in leaves. Plant Physiol. 75:895-901. 101. Norman, H. A. and J. B. St. John. 1987. Differential effects of a substitutedpyridazinone,BAS 13-338, on pathways of monogalac- Volume 39, Issue 3 (July-September) 1991 This content downloaded from 199.7.208.94 on Mon, 1 Apr 2013 14:36:23 PM All use subject to JSTOR Terms and Conditions 447 GRONWALD: LIPIDBIOSYNTHESIS INHIBITORS 102. 103. 104. 105. 106. 107. 108. 109. 110. 111. 112. 113. 114. 115. 116. 117. 118. 119. 120. 121. 448 tosyldiacylglycerol synthesis in Arabidopsis. Plant Physiol. 85: 684-688. Norman, H. A., P. Pillai, and J. B. St. John. 1990. In vitro effects of a substitutedpyridazinone, BASF 13-338, on activity of fatty acid desaturasesfrom soybean chloroplasts.Abstr. Weed Sci. Soc. Am. 30:81. Numa, S. and T. Tanabe. 1984. Acetyl-coenzyme A carboxylase and its regulation. Pages 1-27 in A. Neuberger and L.L.M. Van Deener, eds. Fatty Acid Metabolism and its Regulation. Elsevier, New York. Parker, W. B., D. A. Somers, D. L. Wyse, R. A. Keith, J. D. Burton, J. W. Gronwald, and B. G. Gengenbach. 1990. Selection and characterizationof sethoxydim-tolerantmaize tissue cultures. Plant Physiol. 92:1220-1225. Parker, W. B., L. C. Marshall, J. D. Burton, D. A. Somers, D. L. Wyse, J. W. Gronwald, and B. G. Gengenbach. 1990. Dominant mutations causing alterations in acetyl-coenzyme A carboxylase confer tolerance to cyclohexanedione and aryloxyphenoxypropionate herbicides in maize. Proc. Natl. Acad. Sci. U.S.A. 87: 7175-7179. Rendina, A. R. and J. M. Felts. 1988. Cyclohexanedioneherbicides are selective and potent inhibitors of acetyl-CoA carboxylase from grasses. Plant Physiol. 86:983-986. Rendina, A. R., J. M. Felts, J. D. Beaudoin, A. C. Craig-Kennard, L. L. Look, S. L. Paraskos, and J. A. Hagenah. 1988. Kinetic characterization, stereoselectivity, and species selectivity of the inhibition of plant acetyl-CoA carboxylase by the aryloxyphenoxypropionic acid grass herbicides. Arch. Biochem. Biophys. 265: 219-225. Rendina, A. R., J. D. Beaudoin, A. C. Craig-Kennard,and M. K. Breen. 1989. Kinetics of inhibition of acetyl-coenzyme A carboxylase by the aryloxyphenoxypropionate and cyclohexanedione graminicides. Proc. Brighton Crop Prot. Conf.-Weeds 1:163-172. Rivera, C. M. and D. Penner. 1979. Effect of herbicides on plant cell membrane lipids. Pages 45-76 in F. A. Gunther and J. D. Gunther, eds. Residue Rev. Vol. 70. Springer-Verlag,New York. Sandmann,G., K-J. Kunert, and P. Boger. 1981. Bleaching activity and chemical constitutionof phenylpyridazinones.Pestic. Biochem. Physiol. 15:288-293. Sandmann, G., I. E. Clark, P. M. Bramley, and P. B6ger. 1984. Inhibition of phytoene desaturase-the mode of action of certain bleaching herbicides. Z. Naturforsch. 39c:443-449. Schuphan, I. and J. E. Casida. 1979. S-chloroallyl thiocarbamate herbicides: Chemical and biological formation and rearrangement of diallate and triallate sulfoxides. J. Agric. Food Chem. 27: 1060-1067. Secor, J. and C. Cseke. 1988. Inhibitionof acetyl-CoA carboxylase activity by haloxyfop and tralkoxydim. Plant Physiol. 86:10-12. Secor, J., C. Cseke, and W. J. Owen. 1989. The discovery of the selective inhibition of acetyl coenzyme A carboxylase activity by two classes of graminicides. Proc. Brighton Crop Prot. Conf.Weeds. 1:145-154. Sharp, D. B. 1988. Alachlor. Pages 301-333 in P. C. Kearney and D. D. Kaufman, eds. Herbicides: Chemistry, Degradation, and Mode of Action. Vol. 3. Marcel-Dekker, New York. Shimabukuro,R. H. 1990. Selectivity and mode of action of the postemergence herbicide diclofop-methyl. Plant Growth Regulator Soc. Am. Quart. 18:37-54. Shimabukuro,M. A., R. H. Shimabukuro,and W. C. Walsh. 1982. The antagonism of LAA-induced hydrogen ion extrusion and coleoptile growth by diclofop-methyl. Physiol. Plant. 56:444-452. Shimabukuro, R. H., W. C. Walsh, and R. A. Hoerauf. 1979. Metabolism and selectivity of diclofop-methyl in wild oat and wheat. J. Agric. Food Chem. 27:615-623. Slabas, A. R. and A. Hellyer. 1985. Rapid purification of a high molecular weight subunitpolypeptide form of rape seed acetyl-CoA carboxylase. Plant Sci. 39:177-182. Stanger, C. E. and A. P. Appleby. 1989. Italian ryegrass (Lolium multiflorum)accessions tolerantto diclofop. Weed Sci. 37:350-352. Still, G. G., D. G. Davis, and G. L. Zander. 1970. Plant epicuticular 122. 123. 124. 125. 126. 127. 128. 129. 130. 131. 132. 133. 134. 135. 136. 137. 138. 139. 140. 141. 142. 143. lipids: Alteration by herbicidal carbamates. Plant Physiol. 46: 307-314. St. John, J. B. 1976. Manipulation of galactolipid fatty acid composition with substituted pyridazinones. Plant Physiol. 57: 38-40. St. John, J. B. 1982. Effects of herbicides on the lipid composition of plant membranes. Pages 97-109 in D. E. Moreland, J. B. St. John, and F. D. Hess, eds. Biochemical Responses Induced by Herbicides. Am. Chem. Soc., Washington, DC. St. John, J. B. and J. L. Hilton. 1976. Structureversus activity of substitutedpyridazinonesas related to mechanism of action. Weed Sci. 24:579-582. St. John, J. B., M N. Christiansen,E. N. Ashworth, and W. A. Gentner. 1979. Effect of BASF 13-338, a substitutedpyridazinone, on linolenic acid levels and winterhardinessof cereals. Crop Sci. 19:65-69. Stoltenberg, D. E. 1988. Selectivity and mechanism of action of sethoxydim and haloxyfop. Ph.D. Thesis. Univ. Minnesota. 48 pp. Stoltenberg,D. E., J. W. Gronwald,D. L. Wyse, J. D. Burton,D. A. Somers, and B. G. Gengenbach. 1989. Effect of sethoxydim and haloxyfop on acetyl-coenzyme A carboxylase activity in Festuca species. Weed Sci. 37:512-516. Stang, R. H. and R. L. Rogers. 1974. Behavior and fate of two phenylpyridazinone herbicides in cotton, corn, and soybean. J. Agric. Food Chem. 22:1119-1125. Stumpf,P. K. 1987. The biosynthesis of saturatedfatty acids. Pages 121-136 in P. K. Stumpf, ed. The Biochemistry of Plants. Vol. 9. Lipids: Structure and Function. Academic Press, London. Swisher, B. A. and F. T. Corbin. 1982. Behavior of BAS-9052 OH in soybean (Glycine max) and Johnsongrass (Sorghum halepense) plant and cell cultures. Weed Sci. 30:640-650. Tanabe, T., K. Wada, T. Okazaki, and S. Numa. 1975. Acetylcoenzyme-A carboxylase from rat liver: Subunit structure and proteolytic modification. Eur. J. Biochem. 57:15-24. Turnham,E. and D. H. Northcote. 1983. Changes in the activity of acetyl-CoA carboxylase during rape-seed formation. Biochem. J. 212:223-229. Uchiyama, M., N. Washio, T. Ikai, H. Igarashi, and K. Suzuki. 1986. Stereospecific responses to (R)-(+)-and (S)-(-)-quizalofopethyl in tissues of several plants. J. Pestic. Sci. 11:459-467. Walker, K. A. and J. L. Harwood. 1986. Evidence for separate elongation enzymes for very-long-chain-fatty-acid synthesis in potato (Solanum tuberosum). Biochem. J. 237:41-46. Walker, K. A., S. M. Ridley, and J. L. Harwood. 1988. Effects of the selective herbicide fluazifop on fatty acid synthesis in pea (Pisum sativum) and barley (Hordeum vulgare). Biochem. J. 254: 811-817. Walker, K. A., S. M. Ridley, T. Lewis, and J. L. Harwood. 1988. Fluazifop, a grass-selective herbicide which inhibits acetyl-CoA carboxylase in sensitive plant species. Biochem. J. 254:307-310. Wang, X-M, D. F. Hildebrand,H. A. Norman, M. L. Dahmer, J. B. St. John, and G. B. Collins. 1987. Reduction of linolenate contentin soybean cotyledons by a substitutedpyridazinone. Phytochemistry 26:955-960. Warmund, M. R., H. D. Kerr, and E. J. Peters. 1985. Lipid metabolism in grain sorghum (Sorghum bicolor) treated with alachlor plus flurazole. Weed Sci. 33:25-28. Weisshaar,H. and P. Boger. 1987. Primaryeffects of chloroacetamides. Pestic. Biochem. Physiol. 28:286-293. Weisshaar, H., G. Retzlaff, and P. Boger. 1988. Chloroacetamide inhibition of fatty acid synthesis. Pestic. Biochem Physiol. 32: 212-216. Wilkinson, K E. 1974. Sicklepod surface wax response to photoperiodand S-(2,3-dichloroallyl)-diisopropylthiocarbanate(Diallate). Plant Physiol. 53:269-275. Wilkinson, R. E. 1982. Alachlor influence on sorghum growth and gibberellin precursor synthesis. Pestic. Biochem. Physiol. 17: 177-184. Wilkinson, R. E. 1983. Gibberellinprecursorbiosynthesis inhibition by EPTC and reversal by R-25788. Pestic. Biochem Physiol. 19: 321-329. Volume 39, Issue 3 (July-September) 1991 This content downloaded from 199.7.208.94 on Mon, 1 Apr 2013 14:36:23 PM All use subject to JSTOR Terms and Conditions WEED SCIENCE 144. Wilkinson, R. E. 1986. Diallate inhibition of gibberellin biosynthesis in sorghum coleoptiles. Pestic. Biochem. Physiol. 25:93-97. 145. Wiknson, R. E. 1988. Carbamothioates.Pages 245-300 in P. C. Keamney and D. D. Kaufman, eds. Herbicides: Chemistry, Degradation, and Mode of Action. Vol. 3. Marcel-Dekker, New York. 146. Wilkinson, R. E. and W. S. Hardcastle. 1969. EPTC effects on sicklepod petiolar fatty acids. Weed Sci. 17:335-338. 147. Wilkinson, R. E. and W. S. Hardcastle. 1970. EPTC effects on total leaflet fatty acids and hydrocarbons. Weed Sci. 18:125-128. 148. Wilkinson, R. E. and A. E. Smith. 1975. Thiocarbamateinhibition of fatty acid biosynthesis in isolated spinach chloroplasts. Weed Sci. 23:100-104. 149. Wilkinson, R. E. and A. E. Smith. 1976. Butylate, pebulate, and vernolate inhibition of plant fatty acid biosynthesis. Phytochemistry 15:841-842. 150. Wilkinson, R. E. and D. Ashley. 1979. EPTC induced modification of gibberellin biosynthesis. Weed Sci. 27:270-274. 151. Wilkinson, R. E. and T. H. Oswald. 1987. S-ethyl dipropylthiocarbamate (EPTC) and 2,2-dichloro-N,N-di-2-propenylacetamide (dichlormid) inhibitions of synthesis of acetyl-coenzyme A derivatives. Pestic. Biochem. Physiol. 28:38-43. 152. Willemot, C., C. R. Slack, J. Browse, and P. G. Roughan. 1982. Effect of BASF 13-338, a substituted pyridazinone, on lipid metabolism in leaf tissue of spinach, pea, linseed, and wheat. Plant Physiol. 70:78-81. 153. Winkler,D. A., A. J. Liepa, J. E. Anderson-McKay,and N. K. Hart. 1989. A molecular graphics study of factors influencing herbicidal activity of oximes of 3-acyl-tetrahydro-2H-pyran-2,4-diones. Pestic. Sci. 27:45-63. 154. Wright,J. P. and R. H. Shinabukuro. 1987. Effects of diclofop and diclofop-methyl on the membrane potentials of wheat and oat coleoptiles. Plant Physiol. 85:188-193. 155. Wurtele, E. S. and B. J. Nikolau. 1990. Plants contain multiple biotin enzymes: Discovery of 3-methylcrotonyl-CoAcarboxylase, propionyl-CoA carboxylase and pyruvate carboxylase in the plant kingdom. Arch. Biochem. Biophys. 278:179-186. 156. Yeh, L-A and K-H Kim. 1980. Regulation of acetyl-CoA carboxylase: Properties of CoA activation of acetyl-CoA carboxylase. Proc. Natl. Acad. Sci. U.SA. 7:3351-3355. 157. Yeh, L-A, K-H Lee, and K-H Kim. 1980. Regulation of rat liver acetyl-CoA carboxylase: Regulation of phosphorylationand inactivation of acetyl-CoA carboxylaseby the adenylateenergy charge. J. Biol. Chem. 255:2308-2314. 158. Yenne, S. P. and K. K. Hatzios. 1989. Influence of oxime ether safeners and metolachloron acetate incorporationinto lipids and on acetyl-CoA carboxylase of grain sorghum. Pestic. Biochem. Physiol. 35:146-154. 159. Zama, P. and K. K. Hatzios. 1987. Interactions between the herbicide metolachlor and the safener CGA-92194 at the levels of uptake and macromolecularsynthesis in sorghum leaf protoplasts. Pestic. Biochem. Physiol. 27:86-96. Volume 39, Issue 3 (July-September) 1991 This content downloaded from 199.7.208.94 on Mon, 1 Apr 2013 14:36:23 PM All use subject to JSTOR Terms and Conditions 449