* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download File
Survey
Document related concepts
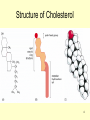
Transcript
Cholesterol Metabolism Dr Nancy Carmichael Thursday 22nd November 2007 1 Objectives • • • • • Roles of cholesterol in the body Basic structure of cholesterol Stages in the synthesis of cholesterol Regulation of cholesterol synthesis Cholesterol as a precursor for bile salts and steroid hormones • Cholesterol transport and the role of LDL • Diseases related to cholesterol metabolism and their treatment 2 Cholesterol • Very important steroid • Has roles in modulating the fluidity of animal cell membranes and is the precursor of steroid hormones such as progesterone, testosterone, oestradiol, and cortisol • It can be consumed in the diet or synthesised de novo • Synthesis and utilization of cholesterol must be tightly regulated in order to prevent overaccumulation and abnormal deposition within the body • Accumulation of cholesterol can lead to atherosclerosis, a disease of the coronary 3 arteries Structure of Cholesterol 4 Cholesterol in the Lipid Bilayer • Eukaryotic plasma membrane has large amounts of cholesterol – up to 1 molecule for every phospholipid molecule • Fills space between phospholipid molecules next to each other • Makes bilayer stiffer, less fluid, and also less permeable 5 Origin of Carbon Atoms in Cholesterol • All 27 carbon atoms in cholesterol come from acetate 1. Label acetate – feed to rats. Cholesterol synthesised by rats contained the label 2. Label acetate on either the methyl or carboxyl carbon 6 Cholesterol Carbon Numbering 7 Synthesis of Cholesterol 1. Stage one is the synthesis of isopentenyl pyrophosphate, an activated isoprene unit that is the key building block of cholesterol. 2. Stage two is the condensation of six molecules of isopentenyl pyrophosphate to form squalene. 3. In stage three, squalene cyclises and is converted to cholesterol 8 9 Stage 1: Synthesis of Isopentenyl Pyrophosphate i. First, 2 acetyl-CoAs form acetoacetyl-CoA ii. Then acetoacetyl-CoA and acetyl-CoA combine to make 3-hydroxy-3methylglutaryl CoA (3-HMG CoA) iii. 3-HMG CoA is then reduced to mevalonate in the cytosol iv. Mevalonate is converted to isopentenyl pyrophosphate 10 (i) 2 acetyl-CoAs form acetoacetylCoA 11 (ii) Formation of 3-HMG CoA 12 (iii) 3-HMG CoA to Mevalonate • 3-HMG CoA has 2 fates: – Conversion to mevalonate for synthesis of cholesterol – cytosol – Cleaved to form acetyl CoA and acetoacetate mitochondria • The synthesis of mevalonate is the committed step in cholesterol formation • The enzyme catalysing this irreversible step, (3-HMG-CoA reductase), is an important control site in cholesterol biosynthesis 13 14 (iv) Mevalonate to Isopentenyl Pyrophosphate • Mevalonate is converted into 3-isopentenyl pyrophosphate in: – three consecutive reactions requiring ATP – a decarboxylation reaction 15 (iv) Mevalonate to Isopentenyl Pyrophosphate 16 (iv) Mevalonate to Isopentenyl Pyrophosphate 17 (iv) Mevalonate to Isopentenyl Pyrophosphate 18 (iv) Mevalonate to Isopentenyl Pyrophosphate 19 Stage 2: Isopentenyl Pyrophosphate to Squalene • Squalene is synthesised from isopentenyl pyrophosphate in reactions involving the following number of carbon atoms: C5 → C10 → C15 → C30 i. isomerisation of isopentenyl pyrophosphate (C5) to dimethylallyl pyrophosphate (C5) ii. isopentenyl pyrophosphate and dimethylallyl pyrophosphate condense to form a geranyl pyrophosphate (C10) (enzyme; geranyl transferase) iii. Geranyl pyrophosphate then combines with isopentenyl pyrophosphate to form farnesyl pyrophosphate (C15) (enzyme: geranyl transferase) iv. two molecules of farnesyl pyrophosphate combine to form squalene (C30) (enzyme: squalene synthase) 20 (i) Isomerisation of Isopentenyl Pyrophosphate to Dimethylallyl Pyrophosphate 21 (ii) Formation of Geranyl Pyrophosphate 22 (iii) Formation of Farnesyl Pyrophosphate 23 (iv) Formation of Squalene 24 Stage 3: Squalene forms Cholesterol i. First stage, squalene to squalene epoxide, is a reduction reaction requiring O2 ii. Squalene epoxide is cyclised to lanosterol by a cyclase enzyme • • Migration of 2 methyl groups movement of electrons through 4 double bonds iii. Lanosterol is converted to cholesterol by: • • • removal of 3 methyl groups reduction of a double bond by NADPH migration of another double bond 25 (i) Squalene to Squalene Epoxide 26 (ii) Squalene Epoxide to Lanosterol • Migration of 2 methyl groups • Movement of electrons through 4 double bonds 27 (iii) Lanosterol to Cholesterol HCOOH + 2CO2 • Removal of 3 methyl groups • Reduction of one double bond by NADPH • Migration of another double bond 28 Summary of Cholesterol Biosynthesis 29 Regulation of Cholesterol Synthesis • Cholesterol can be obtained from the diet or it can be synthesised de novo • The liver is the major site of cholesterol synthesis in mammals, although the intestine also forms significant amounts • The rate of cholesterol formation by these organs is very responsive to the cellular level of cholesterol • This is called feedback regulation • Here, it is mediated mostly by changes in the amount and activity of 3-HMG CoA reductase • 3-HMG-CoA reductase is controlled in many ways 30 HMG CoA Reductase Regulation 1. Rate of synthesis of 3-HMG CoA reductase mRNA 2. Rate of translation of 3-HMG CoA reductase mRNA into protein 3. Degradation of 3-HMG CoA reductase 4. Phosphorylation of 3-HMG CoA reductase 31 HMG CoA Reductase Regulation DNA 1 mRNA 2 protein 3 degradation products 4 protein P 32 1.Synthesis of HMG CoA Reductase mRNA • The rate of synthesis of reductase mRNA is controlled by the sterol regulatory element binding protein (SREBP) • This transcription factor binds to a short DNA sequence called the sterol regulatory element (SRE) on the 5’ side of the reductase gene • In the presence of sterols, SRE inhibits mRNA production 33 2. Translation of HMG CoA Reductase mRNA • The rate of translation of reductase mRNA is inhibited by nonsterol metabolites derived from mevalonate, as well as by dietary cholesterol. 34 3. Degradation of HMG CoA Reductase • The enzyme is bipartite: – cytosolic domain carries out catalysis – membrane domain senses levels of derivatives of cholesterol and mevalonate • A high level of these products leads to rapid degradation of the enzyme 35 4. Phosphorylation of HMG CoA Reductase • Phosphorylation decreases the activity of the reductase • Hormones regulate phosphorylation: – Glucagon stimulates phosphorylation (deactivation) – Insulin stimulates dephosphorylation (activation) 36 Negative Feedback of Cholesterol Synthesis • All four regulatory mechanisms are modulated by receptors that sense the presence of cholesterol in the blood • Negative feedback inhibition 37 Fates of Cholesterol • Most cholesterol synthesis takes place in liver • Some of this is incorporated into membrane of liver cells • Most is exported in the forms of: – Bile acids and their salts – Cholesteryl esters • Cells use the cholesterol for membrane synthesis • They can also use it as a precursor for steroid hormone production and vitamin D production 38 Bile Acids and Salts • Bile acids and salts are derivatives of cholesterol • Make good detergents as they contain polar and non-polar regions • They are synthesised in the liver • They are the main constituent of bile • They emulsify dietary lipids, which increases their surface area to: – Promote hydrolysis by lipases – Facilitates their absorption by the intestine • Bile salts also aid in absorption of lipid soluble vitamins 39 Bile Salts as an Emulsifier 40 Excretion of Cholesterol in Bile • Synthesis of bile acids is one of the predominant mechanisms for the excretion of excess cholesterol • However, the excretion of cholesterol in the form of bile acids is insufficient to compensate for an excess dietary intake of cholesterol. 41 Synthesis of Bile Acids • Bile acids are synthesised from cholesterol via many reactions • The first reaction, cholesterol to 7ahydroxycholesterol (enzyme: 7a-hydroxylase) is the rate limiting step in bile acid synthesis • Conversion of 7a-hydroxycholesterol to the bile acids requires several steps (not shown in diagram) 42 43 Bile Acids • The most abundant bile acids in human bile are chenodeoxycholic acid and cholic acid • These are the primary bile acids • In intestines, primary bile acids are converted to the secondary bile acids – deoxycholate (from cholate) – lithocholate (from chenodeoxycholate) 44 Synthesis of Bile Salts • In the liver the carboxyl group of primary and secondary bile acids is conjugated via an amide bond to either glycine or taurine • React with glycine to form glycocholate • React with taurine (a derivative of cysteine: H2N-CH2-CH2-SO3-) to form taurocholate • Glycocholate is the main bile salt • They are secreted into the intestine, where they aid in the emulsification of dietary lipids 45 Synthesis of Bile Salts • Glycocholiate (glycocholic acid) • Taurocholate (taurocholic acid) 46 Cholesterol used to Synthesise Steroids • Cholesterol is the precursor of the five major classes of steroid hormones: – progestagens – glucocorticoids – mineralocorticoids – androgens – oestrogens • These hormones are powerful signal molecules that regulate many processes in the body 47 Cholesterol and Steroid Hormones Cholesterol (C27) Pregnelonone (C21) Progestagens (C21) Mineralocorticoids Androgens (C19) (C21) Glucocorticoids (C21) Oestrogens (C 48 19) Cholesteryl Esters • About 2/3 of cholesterol in blood is in form of an ester • Cholesteryl esters are formed in the liver via the action of acyl-CoA-cholesterol acyl transferase (ACAT) • Catalyses transfer of a fatty acid from coenzyme A to the hydroxyl group of cholesterol • This changes cholesterol into a more hydrophobic form • Can be stored in the liver or transported to other tissues which need cholesterol 49 50 Transport of Cholesterol in the Blood • • • • • Cholesterol and cholesteryl esters are virtually insoluble in water They need to be transported from origin to other tissues for storage or use They are transported in body fluids in the form of lipoprotein particles These are complexes of an apolipoprotein combined with cholesterol, cholesteryl esters, phospholipids or triacylglycerol Apolipoprotein is the protein in its lipid free 51 form Transport of Cholesterol in the Blood • • Different combination of apolipoproteins and lipids produce particles of different density Lipoproteins are classified according to increasing density – – – – – – Chylomicrons Chylomicron remnants Very low density lipoproteins (VLDL) Intermediate-density lipoproteins (IDL) Low density lipoproteins (LDL) High density lipoproteins (HLDL) 52 Low Density Lipoprotein 53 54 Specific Function of Lipoproteins • Each class of lipoproteins has a specific function • This depends on its point of synthesis, lipid composition and apolipoprotein content • At least 9 apolipoproteins are found in lipoproteins of human plasma • The apolipoprotein has 2 roles: 1.Solubilise hydrophobic lipids 2.Contain targeting signals – target lipoprotein to a specific tissue or enzyme 55 Chylomicrons • Largest, least dense, contain many triacylglycerols • Synthesised in endoplasmic reticulum of epithelial cells in small intestine, then enter blood stream • Carry fatty acids to where they are used or stored • The chylomicron remnants (without fats but still containing cholesterol) move to liver • apoE in the chylomicron remnants bind receptors in liver – taken up by endocytosis • In liver, they release cholesterol and are degraded in lysosomes 56 VLDL • If diet has more fatty acids than needed – converted to triacylglycerols in liver and packaged into VLDL • Excess carbohydrate can also be dealt with in the same way • VLDLs are transported in the blood from liver to muscle and adipose tissue • Here the fatty acids are relased from the triacylglycerols in VLDL • Adipocytes take up the fatty acids and resynthesise triacylglycerols to store • Most VLDL remnants (IDL) removed from circulation by hepatocytes: are uptaken by endocytosis (if apoE present) • Half the VLDL become LDL due to loss of lipids 57 58 Chylomicrons give plasma a milky appearance 59 LDL • Loss of triacylglycerols converts some VLDL to VLDL remnants (IDL) and then to LDL • LDLs are very rich in cholsterol and cholesteryl esters • They have apoB-100 as the major apolipoprotein • They carry cholesterol to extrahepatic tissue which has receptors to recognise apoB-100 • The receptors mediate the uptake of cholesterol and cholesteryl esters 60 HDL • These begin in liver and small intestine • Contains very little cholesterol and no cholesteryl esters • Very protein rich • Contain enzyme LCAT (lecithin-cholesterol acyl transferase) • LCAT catalyses formation of cholesteryl esters from lecithin (phosphatidylcholine) and cholesterol 61 Formation of Cholesteryl Esters from Lecithin 62 HDL and LCAT • LCAT is on the surface of nascent (newly forming) HDL particles • It converts the cholesterol and lecithin of the chylomicron and VLDL remnants to cholesteryl esters • These form a core and makes HDL molecules from disc shaped to spherical • HDL particles are then mature • This cholesterol-rich lipoprotein then returns to the liver where it unloads some cholesterol which is converted to bile salts 63 HDL • HDL may be taken up by the liver by receptor mediated endocytosis • Some is taken to other tissues • HDL binds to plasma membrane receptor proteins (SR-BI) in hepatic and steroidogenic tissues e.g. adrenal gland • These mediate the selective transfer of cholesterol and other lipids in HDL into the cell • The depleted HDL then dissociates to recirculate in the blood stream and extract more lipids from chylomicron and VLDL remnants 64 Reverse Cholesterol Transport • Depleted HDL can also pick up cholesterol stored in extrahepatic tissues and carry it to the liver • This is called reverse cholesterol transport • In one reverse cholesterol transport pathway, interaction of nascent HDL with SR-BI receptors in cholesterol-rich cells triggers passive movement of cholesterol from the cell surface into HDL • HDL then carries it back to liver 65 Reverse Cholesterol Transport • Another reverse cholesterol transport pathway – apoA-I in depleted HDL interacts with an active transporter in a cholesterolrich cell • This is the ABC1 protein • The apoA-I and the HDL are taken up by endocytosis • They are then re-secreted with a load of cholesterol • It transports this back to the liver 66 Receptor Mediated Endocytosis • LDL – apoB-100, recognised by LDL receptors • LDL receptors are present on cells which need to take up cholesterol • LDL binding to its receptor initiates endocytosis • This brings the LDL and its receptor iside the cell, within an endosome • This endosome fuses with a lysosome • The lysosome contains enzymes which hydrolyse the cholesteryl esters to cholsterol and free fatty acids • apoB-100 is degraded to amino acids 67 Endocytosis of LDL 68 Receptor Mediated Endocytosis • LDL also degraded to amino acids which are released into cytosol • LDL receptor is not degraded – it returns to the cell surface • apoB-100 present in VLDL, but it cannot bind to the LDL receptor • Conversion of VLDL to LDL exposes the binding domain of apoB-100 69 70 ACAT • Cholesterol which enters cell by receptor mediated endocytosis can be re-esterified by ACAT (acyl-CoA-cholesterol acyltransferase) • Stored within cytosolic lipid droplets 71 Regulation of Blood Cholesterol • Accumulation of excess cholesterol prevented by reducing rate of cholesterol synthesis when sufficient cholesterol is available from blood LDL • Negative feedback inhibition • High intracellular cholesterol levels activate ACAT, increases esterification of cholesterol for storage • High cellular cholesterol reduces transcription of the gene which encodes LDL receptor • Reduces production of receptor and thus uptake of cholesterol from blood 72 73 Unregulated Cholesterol Production • If more cholesterol is consumed in diet and synthesised than is needed for synthesis of membranes, bile salts and steroids: – accumulation of cholesterol in blood vessels – These are called atherosclerotic plaques – Obstruct blood flow leading to atherosclerosis 74 Atherosclerosis • Blocked vessels, occluded coronary arteries – heart attacks • Major cause of death in industrialised societies • Atherosclerosis linked to high blood cholesterol • Also to high levels of LDL-bound receptor • Negative correlation between HDL and arterial disease 75 Familial Hypercholesterolemia • • • • • Genetic disorder High blood cholesterol Develop atherosclerosis in childhood Defective LDL receptor Receptor mediated uptake of cholesterol by LDL does not occur • Cholesterol is not cleared from blood • Accumulated forming atherosclerotic plaques • Endogenous cholesterol synthesis continues despite excessive cholesterol in blood because extracellular cholesterol cannot enter cell to regulate intracellular synthesis 76 Treating Familial Hypercholesterolemia • Lovastatin and Compactin – derived from fungi • These, and other analogues resemble mevalonate • Competitive inhibitors of HMG-CoA reductase • Inhibit cholesterol synthesis • Lovastatin can lower blood cholesterol up to 30 % • Can combine drug with an edible resin which binds bile salts to prevent their re-absorption – makes drugs more effective 77 78 HDL Deficiency • • • • • • • • HDL levels low in familial HDL deficiency (FHA) HDL almost undetectable in Tangier disease Both result from a mutation in ABC protein Cholesterol depleted HDL can’t take on cholesterol from cells that lack this protein The cholesterol depleted HDL is rapidly removed from the blood and destroyed Both diseases rare Teach us about role of ABC1 protein Low plasma HDL correlates with high incidence of coronary heart disease - ABC1 useful target for drugs 79 to control HDL levels Intermediates in Cholesterol Biosynthesis • Isopentenyl pyrophosphate has other roles apart from in cholesterol synthesis • Precursor for synthesis of many biomolecules • Vitamins A,E and K, natural rubber, essential oils and many others • These compounds are called isoprenoids 80 Isoprenoid Biosynthesis 81 Practice Questions 1. Which of the following is not an intermediate in the synthesis of lanosterol from acetyl-CoA? a) b) c) d) e) Isopentenyl pyrophosphate Malonyl-CoA Mevalonate Squalene HMG-CoA 2. Cholesterol is synthesised from: a) b) c) d) e) acetyl-CoA choline lipoic acid malate oxalate 82 Practice Questions 3. A 30-carbon precursor of the steroid nucleus is: a) b) c) d) e) farnesyl pyrophosphate. geranyl pyrophosphate. isopentenyl pyrophosphate. lysolecithin. squalene. 4. Which of the following is derived from a sterol? a) b) c) d) e) Bile salts Gangliosides Geraniol Phosphatidylglycerol Prostaglandins 83 Practice Questions 5. Which of these statements about cholesterol synthesis is true? a) Cholesterol is the only known natural product whose biosynthesis involves isoprene units. b) Only half of the carbon atoms of cholesterol are derived from acetate. c) Squalene synthesis from farnesyl pyrophosphate results in the release of two moles of PPi for each mole of squalene formed. d) The activated intermediates in the pathway are CDPderivatives. e) The condensation of two five-carbon units to yield geranyl pyrophosphate occurs in a “head-to-head” fashion. 84 Practice Questions 6. Which of these statements about the regulation of cholesterol synthesis is not true? a) Cholesterol acquired in the diet has essentially no effect on the synthesis of cholesterol in the liver. b) Failure to regulate cholesterol synthesis predisposes humans to atherosclerosis. c) High intracellular cholesterol stimulates formation of cholesterol esters. d) Insulin stimulates HMG-CoA reductase. e) Some metabolite or derivative of cholesterol inhibits HMG-CoA reductase. 85 Practice Questions 7. What are the functions of cholesterol in humans? 8. Which is the commited step in cholesterol synthesis? What enzyme catalyses this step? 9. How are the 2 activated isoprenes structurally related? 10. Which are the most dense lipoproteins? The least dense? How does this relate to their lipid:protein ratio? 11. What part of the LDL particle is recognised by the LDL receptor? 12. Based on the function of HDLs, why do you expect a negative correlation between HDL levels and arterial disease? 86