* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Cytochrome P450-enzymes involved in the biosynthesis of mono
Survey
Document related concepts
Evolution of metal ions in biological systems wikipedia , lookup
Expression vector wikipedia , lookup
Plant virus wikipedia , lookup
Light-dependent reactions wikipedia , lookup
Catalytic triad wikipedia , lookup
Enzyme inhibitor wikipedia , lookup
Plant nutrition wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Oxidative phosphorylation wikipedia , lookup
Plant breeding wikipedia , lookup
Biochemistry wikipedia , lookup
Metalloprotein wikipedia , lookup
Amino acid synthesis wikipedia , lookup
Biosynthesis of doxorubicin wikipedia , lookup
Transcript
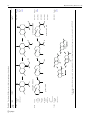
Phytochem Rev (2015) 14:7–24 DOI 10.1007/s11101-013-9280-x Cytochrome P450-enzymes involved in the biosynthesis of mono- and sesquiterpenes Corinna Weitzel • Henrik Toft Simonsen Received: 29 August 2012 / Accepted: 5 March 2013 / Published online: 17 March 2013 Ó Springer Science+Business Media Dordrecht 2013 Abstract Terpenoids form the largest group of plant specialized metabolites and exhibit essential functions in plant metabolism, propagation and defence. Since several mono- and sesquiterpenoids, like artemisinin, menthol and nootkatone, have proven beneficial for mankind, they also possess high socio-economic value. The general mechanisms of terpene biosynthesis are understood and enzymes catalysing the formation of the isoprenoid basic carbon skeletons have been described frequently. In the subsequent pathway steps, it is mainly cytochromes P450 that catalyse the decoration of these basic skeletons and thereby contribute significantly to the structural diversity observed. Structure–function relationship, even though discussed intensively, is poorly understood for this enzyme family; even with the phylogenic relationship well established identification of the functionality of the single enzymes is challenging, and, so far, only a few have been characterized. This review provides an overview over cytochromes P450 participating in the biosynthesis of mono- and sesquiterpenes. Only enzymes that have been described thoroughly after purification and heterologous expression are included in this review and their characteristic features are discussed. C. Weitzel H. T. Simonsen (&) Department of Plant and Environmental Sciences, Faculty of Science, University of Copenhagen, Thorvaldsensvej 40, 1871 Frederiksberg C, Denmark e-mail: [email protected] Keywords Cytochrome P450 Isoprenoids Plant specialized metabolism Terpenoids Abbreviations CPR Cytochrome P450 reductase CYP Cytochrome P450 GAO Germacrene acid oxidase SRS Substrate recognition site TPS Terpene synthase Introduction Plant cells are capable of forming an overwhelming variety of specialized metabolites, both in terms of complexity and quantity. These small organic molecules allow plants to cope with various types of stress, and often have biological activities beneficial to humans that make them of high commercial interest to the pharmaceutical and biotechnological industry (Cragg et al. 2011). Many of these biologically active compounds are terpenoids. With several tens of thousands known structures, terpenoids form the largest class of plant natural products (Buckingham 2011). Some terpenoids are key constituents for cellular functions (e.g., electron transport chains, steroidal membrane components, and hormones), but many of them are specialized metabolites that influence the fitness of the synthesizing organism (Ashour et al. 2010). Plant terpenoids are known to serve as insect and herbivore deterrents, 123 8 allochemicals, and as attractants for pollinators and beneficial insects in tritrophic interactions (Gershenzon and Dudareva 2007). As a result, a universal statement about spatial and temporal patterns of accumulation is not possible (Gershenzon et al. 2000). In some plants, terpenoids are synthesized in specialized physical structures such as oil glands or resin ducts (Miller et al. 2005), whereas in others biosynthesis is not even restricted to particular organs but proceeds basically in all tissues (Bohlmann and Keeling 2008). Since many terpenoids serve as defence compounds, their biosynthesis is often induced by stress, and regulation seems mainly to take place at transcriptional level (Crocoll 2011). Besides their eco-physiological role, terpenoids have also benefited mankind as flavours, fragrances, pharmaceuticals, nutraceuticals, and industrial chemicals (Berger 2007; Zwenger and Basu 2008). Biotechnological production is of special interest for terpenoids with known pharmacological properties or with application in foods and fragrances, since their limited availability in nature and the structural complexity of the molecules can make it the only commercial sustainable method of production (Daviet and Schalk 2010). This however requires profound knowledge on the presence of terpenoids in plants and their biosynthesis (Simonsen et al. 2009). The wide range of physiological activities and industrial applications exhibited by terpenoids can be attributed to their structural diversity that is a result of the catalytic plasticity of terpene synthases (TPSs). TPSs convert simple linear prenyldiphosphates into complex terpenes, often with cyclic groups and a large number of chiral centres (Bohlmann et al. 1998). Following the core terpenoid formation further modifications are often catalysed by cytochromes P450 often leading to oxidations of the terpenoid. Additionally, also dehydrogenases and acyltransferases have been shown to contribute to the structural diversity of terpenes (Ashour et al. 2010), furthermore methylation, acylation, and glycosylation of terpenoids are described (Mau and Croteau 2006). In this review, we aim to provide an overview of cytochromes P450 involved in mono- and sesquiterpene biosynthesis. The focus is only on those enzymes that have been characterized through cloning and expression. Examinations where only whole cell extracts, microsomes or likewise have been studied are not included due to the complexity of these system, thus leading to results 123 Phytochem Rev (2015) 14:7–24 of less clarity. Along with in vitro and heterologous characterization, in planta characterization should be pursued for all cytochromes P450. Cytochromes P450 form one of the largest and oldest gene superfamilies and can be found in the genome of all biological kingdoms (Nelson 2011). The result of a cytochrome P450 catalysed reaction is most often insertion of oxygen (hydroxylation), although also dehydrogenation, isomerization, dimerization, carbon–carbon bond cleavage, reductions, as well as N-, O- and S-dealkylations, sulphoxidations, epoxidations, deaminations, desulphurations and several more have been described (Bernhardt 2006; Guengerich 2001; Sono et al. 1996). The term ‘P450’ is not based on a common enzymatic function, but their shared ability to display a typical absorption band at 450 nm when carbon monoxide is bound to the reduced form of this b-type heme (Omura and Sato 1964). Based on their amino acid sequence, cytochromes P450 are classified into families (minimum 40 % sequence identity) and subfamilies (greater than 55 % sequence identity) (Werck-Reichhart and Feyereisen 2000). Until summer 2012, a nomenclature committee led by Prof David Nelson, The University of Tennessee Health Science Center, named over 18,000 genes attributed to several hundred families (Nelson 2012). Generally, a common overall topology and tridimensional fold of all cytochromes P450 have been observed (Graham and Peterson 1999) despite their relative low sequence similarity on amino acid level, which can be less than 20 % even within a plant species (Bak et al. 2011). Only a few amino acids are strictly conserved. These are found in the core where they form a four-helix (D, E, I and L) bundle, two sets of b-sheets, and a coil, generally known as the ‘meander’. The catalytic centre consists of a central heme-group bound to the thiolate of a highly conserved cysteine residue (Hasemann et al. 1995; Peterson and Graham 1998). This structural conservation allows for a common reaction mechanism of electron and proton transfer followed by oxygen activation, which has been described in detail by Hamdane et al. (2008). In contrast, little is known about substrate specificity and type of reaction catalysed by the individual enzymes, since these are controlled by less conserved regions of the protein (Werck-Reichhart and Feyereisen 2000). Phytochem Rev (2015) 14:7–24 Determination of candidate genes and investigation of enzyme features Within plants, cytochromes P450 take part in numerous processes of general and specialized metabolism and thus play important roles in the biosynthesis of plant hormones, in cell wall biosynthesis as well as in the biosynthesis of specialized compounds involved in defence or for the attraction of pollinators, to mention just a few examples (Mizutani 2012). Hence, they are abundantly present in genomes and transcriptomes of all plants. So far, 127 plant cytochrome P450-families have been described. The latest plant cytochrome P450-family was added in 2005, implying that the diversification of plant cytochromes P450 seems to be widely covered; the appearance of new subfamilies, however, still continues (Nelson and Werck-Reichhart 2011). The discovery of cytochromes P450 catalysing hydroxylation steps in the biosynthesis of mono- and sesquiterpenes is impeded by many factors that concern determination of the right candidate gene and investigation of the enzyme’s features. Structure– function relationship of plant cytochromes P450 is still poorly understood, and, for this reason, has not lead to substrate discovery. On the other hand, phylogeny could prove a promising approach to limit the possible enzyme functions. Even though enzymatic function of many cytochromes P450 has not been revealed, it has generally been recognized that cytochromes P450 in the same family or subfamily catalyze similar reactions or are involved in the same biosynthetic pathway (Nelson and Werck-Reichhart 2011). Until now, cytochromes P450 contributing to the metabolism of specialized terpenoids are described in 4 plant clans (Hamberger and Bak 2013). This finding indicates that the functions of CYP clans and families are more often a result of evolution than of sequence relatedness (Nelson and Werck-Reichhart 2011), and especially within the CYP71 family, species-specific rather than conserved functions exist (Devi et al. 2011). So far, most terpenoid related cytochromes P450 are members of the CYP71 clan (Fig. 1), a huge clan that comprises cytochromes P450 involved in the metabolism of the majority of specialized compounds (Hamberger and Bak 2013). First, it has mainly been genes from the CYP71A and CYP71D subfamilies that have been associated with oxidation of mono- or 9 sesquiterpenes in different plant species of various families (Hallahan et al. 1994; Mau and Croteau 2006; Ralston et al. 2001; Takahashi et al. 2007), but lately, many new CYP71-subfamilies have been described, many representing enzymes catalysing oxygenation of small molecules, also sesquiterpenoids (Nelson and Werck-Reichhart 2011). Exceptional is CYP72A1 that catalyses formation of secologanin, a monoterpenoid and precursor of the indole alkaloid strictosidine (Irmler et al. 2000; Yamamoto et al. 2000). Members of the same subfamily, CYP72A, take part in triterpene biosynthesis, other CYP72-families are responsible for the catabolism of di- and triterpenoid phytohormones (reviewed by Hamberger and Bak 2013). Discovery of pathway specific cytochromes P450 and their involvement in terpenoid biosynthesis require thorough biochemical characterization. Generally, high substrate specificity of the enzymes has been noticed (Schuler and Werck-Reichhart 2003), hampering this task exceedingly. Accessibility to a certain substrate can be difficult, because many are not commercially available. Several simple monoterpenoids can by chemical synthesis. But for many highly decorated monoterpenoids and most sesquiterpenoids, chemical synthesis can be laborious, inefficient and costly, if possible at all, due to the complex chemical structures. Co-expression of the candidate genes with genes upstream in the proposed pathway can circumvent this problem and is performed more and more often (Greenhagen et al. 2003; Ikezawa et al. 2011); efficient expression of all genes and transcription into functional enzymes in vitro must be ensured and indepth biochemical characterization of all novel enzymes is needed. Recently, various cytochromes P450 capable of accomplishing successive hydroxylation steps were described. These enzymes do not only introduce a hydroxyl group, but further catalyse its subsequent oxidation into a ketone or carbonyl group; some even accept various terpenes, alcohols and aldehydes as substrates. This is still a subject that needs further attention, but it seems to indicate the possibilities for broader substrate acceptance and a wider product portfolio. With this review we aim to establish a current overview and enable further investigation into terpenoid biosynthesis and how cytochromes P450 play a role in this. 123 10 Phytochem Rev (2015) 14:7–24 Fig. 1 A phylogenetic tree showing the cytochromes P450 discussed in this review Cytochromes P450 involved in monoterpene biosynthesis Monoterpenoids have been detected in more than 2000 plant species throughout the plant kingdom and, so far, more than 3,400 different monoterpenoids have been described (Buckingham 2011). The arrangement of the carbon skeleton can be acyclic, mono- and bicyclic. Tricyclic monoterpenoids exist, but they are rare. Monoterpenoids are the major components of most plant essential oils (Evans 2009). In many plant species, essential oil is produced and stored in specialized cells, glandular trichomes, oil glands etc. (Evans 2009). In some cases, these cells could be isolated and served as an enriched source of biosynthetic enzymes and their corresponding transcripts (Mau and Croteau 2006). In 1992, the function of the first plant cytochrome P450, CYP71A1, was described (see Table 1). The research group headed by Dr. Wallsgrove had isolated it from the mesocarp of ripe avocado fruits (Persea 123 americana, Lauraceae) and demonstrated its ability of hydroxylation of the monoterpenoids nerol and geraniol. Surprisingly, neither monoterpenoids nor derived products have been found in the original tissue (Hallahan et al. 1992). Subsequently, various studies have shown that CYP71A1 is also capable of binding a number of substrates; for instance, it carries out a monooxygenation reaction on p-chloro-N-methylaniline (pCMA). The function of the enzyme in vivo, however, remains unclear (Hallahan et al. 1994). Southern blot analysis revealed the existence of a closely related cytochrome P450 expressed in catmint (Nepeta racemosa, Lamiaceae) leaves, this enzyme also catalyses the hydroxylation of nerol and geraniol. The catmint cytochrome P450 specifically hydroxylates both monoterpenes at C10, while CYP71A1 was shown to catalyse 2,3- or 6,7-epoxidation (Hallahan et al. 1994). CYP71A5 and CYP71A6 were eventually cloned from N. racemosa leaves. Participation of CYP71A5 in monoterpene biosynthesis, probably as geraniol 10-hydroxylase, was deduced from expression 71A32 71A6 71A5 71A1related Persea americana 71A1 (Lamiaceae) Mentha x piperita (Lamiaceae) Nepeta racemosa (Lamiaceae) Nepeta racemosa (Lauraceae) Plant origin Cytochrome P450 OH OH OH OH OH nerol 10-hydroxy-nerol geraniol 10-hydroxy-geraniol Proposed function: geraniol-10-hydroxylase; no functional characterization after heterologous expression OH Catalysed reaction Table 1 Cytochrome P450 genes involved in monoterpene biosynthesis AF346833 Y09424 Y09423 M32885 Gen Bank number Bertea et al. (2001), Mizutani and Sato (2011) Clark et al. (1997) Hallahan et al. (1994) Hallahan et al. (1992, 1994) References Phytochem Rev (2015) 14:7–24 11 123 123 Fragaria vesca 71AR1 Mentha spicata 71D18 71D174 (Lamiaceae) 71D15 (Lamiaceae) Perilla frutescens (Lamiaceae) Mentha x piperita 71D13 (Rosaceae) Plant origin Cytochrome P450 Table 1 continued Catalysed reaction GQ120438 AF124815 AF124817 AF124816 Sequence published in reference only Gen Bank number Mau et al. (2010) Haudenschild et al. (2000), Lupien et al. (1999) Haudenschild et al. (2000), Lupien et al. (1999) Aharoni et al. (2004) References 12 Phytochem Rev (2015) 14:7–24 72A1 Thymus vulgaris 71D178182 (Apocynaceae) Catharanthus roseus (Lamiaceae) Origanum vulgare Plant origin Cytochrome P450 Table 1 continued HO OH O O HO In addition, a-terpinene, (?)-R-limonene, (-)-S-limonene and (-)-R-a-phellandrene are converted -terpinene Catalysed reaction thymol carvacrol OH Irmler et al. (2000), Yamamoto et al. (2000) Crocoll (2011) Sequences awaiting publication AAA33106 References Gen Bank number Phytochem Rev (2015) 14:7–24 13 123 Collu et al. (2001) ACZ48680 CAC80883 (Gentianaceae) Swertia mussotii 76B10 Catharanthus roseus 76B6 123 (Apocynaceae) Plant origin Cytochrome P450 Table 1 continued Catalysed reaction Gen Bank number Wang and Essenberg (2010) Phytochem Rev (2015) 14:7–24 References 14 profiles in different leaf tissues and at different stages of development. Nevertheless, enzymatic activity has never been shown after heterologous expression (Clark et al. 1997). Geraniol also serves as a precursor for the biosynthesis of iridoid monoterpenoids and indole alkaloids (El-Sayed and Verpoorte 2007; O’Connor and Maresh 2006; O’Connor and McCoy 2006). In connection with these specialized plant metabolites, members of the subfamily CYP76B, often wrongly named as geraniol 10-hydroxylases, have been extensively studied in Catharanthus roseus (CYP76B6) (Collu et al. 2001; Sung et al. 2011) and Swertia mussotii (CYP76B10) (Wang et al. 2010) and have been demonstrated to catalyse the formation of 8-hydroxygeraniol (not 10-hydroxy-geraniol). Although Arabidopsis thaliana CYP76C10 has been granted a patent as plant geraniol/nerol-8-hydroxylase (US patent No. 5753507) (Bak et al. 2011; Mizutani et al. 1997), true function of the enzyme remains uncertain since neither 8-hydroxygeraniol nor iridoid derived secondary products have been detected in vivo, yet. Lately, Sung and co-workers demonstrated flavonoid 30 -hydroxylase function of CrCYP76B6; the enzyme is able to specifically convert naringenin into eridictyol (Sung et al. 2011). This finding was not only unexpected because of the low sequence identity with other flavonoid 30 -hydroxylases (35–37 % identity on amino acid level), but also because of the different locations of the biosynthesis of flavonoids and monoterpenoids in planta (Kaltenbach et al. 1999; Oudin et al. 2007). Since the conversion of geraniol proceeds approximately 10-times faster than naringenin’s and Km values for geraniol are lower (Sung et al. 2011), geraniol is likely to be the enzyme’s natural substrate. The enzyme’s ability to transform naringenin might solely be a consequence of the flexibility of the substrate binding pocket, which is observed in vitro, and thus reflect the high degree of function promiscuity that has been reported in both CYP76B and CYP71A-families (Bozak et al. 1992; Hamberger and Bak 2013; Robineau et al. 1998). Monoterpenoid biosynthesis and cytochromes P450 involved therein have been studied in-depth in limonene biosynthesis in plants belonging to the mint family (Lamiaceae). Both peppermint (Mentha x piperita), spearmint (M. spicata), and perilla (Perilla frutescens) express limonene hydroxylases, which regiospecifically hydroxylate S-(-)-limonene at C3 Phytochem Rev (2015) 14:7–24 (CYP71D13 and CYP71D15), C6 (CYP71D18) (Lupien et al. 1999) or C7-position (CYP71D174) (Mau et al. 2010), respectively, thereby affording (-)-E-isopiperitenol, (-)-E-carveol or perillyl alcohol. High sequence identity between enzymes (70 %) and the utilization of the same substrate allowed for comprehensive studies of structure–function relationship (Schalk and Croteau 2000). The investigations revealed that only a single amino acid controls regiospecificity of CYP71D18, namely F363. Substitution with isoleucine converted the enzyme into a regiospecifically conserved 6-hydroxylase. Reciprocal mutation of the 3-hydroxylase (CYP71D15), however, did not lead to a catalytically functional enzyme (Schalk and Croteau 2000). F363 is located 5 amino acids downstream of the highly conserved ExxR-motif (see Figure 2 in Mau et al. 2010) in substrate recognition site (SRS) 5 in the loop between the K-helix and the b-sheet 1–4. Together with SRS 1 and helix I, SRS 5 directly flanks the substrate binding cavity. Recently, a different study systematically investigated SRS 5 of more than 6,300 sequences (Seifert and Pleiss 2009). Independent of Schalk and Croteau’s finding, also this study disclosed the existence of a single amino acid residue that directs substrate- and heme-interaction. It is situated at position 5 after the ExxR-motif and is often highly hydrophobic (Seifert and Pleiss 2009; Sirim et al. 2010). Since this motif faces towards the heme centre, it can be expected to interact with all substrates during oxidation (Seifert and Pleiss 2009). Thus, this position is of significant interest for investigations with regards to structure– function relationship of cytochromes P450 involved in small molecule metabolism, and also for terpenoid biosynthesis. In other studies, structural analogues of the substrate S-(-)-limonene were used to analyse active site interactions of CYP71D15 and CYP71D18. Besides the wild types also all active mutants and chimera exclusively provided either C3- or C6-specificity. This implies that shape and rigidity of the active sites restrict substrate orientation severely. Concerning the structure of substrate analogues, investigations demonstrated that a methyl group at C1, bulkier substituents at C4, and additional carbon atoms at either C7 or C9 of limonene hamper catalytic activity. Thus, deviation from the native limonene structure increases loss of regioselectivity of CYP71D18 thereby suggesting steric interaction with the isopropenyl group in 15 the natural substrate to be critical for the orientation in the active site (Wust and Croteau 2002; Wust et al. 2001). In contrast, CYP71D174 cloned from Perilla frutescens shows less limitations in its product profile; under optimal conditions, the enzymes hydroxylates limonene affording a mixture of perillyl alcohol, E-isopiperitenol and E-carveol. Consistent with CYP71D18, CYP71D174 has a phenylalanine in the position corresponding to the critical F363 discussed before; a similar orientation of the substrate in the active site was therefore assumed. Functional characterization of the heterologously expressed enzyme, however, demonstrated that hydroxylation at C7 is preferred upon C3 and C6, thus substrate orientation must be controlled in a more complex way than by a single amino acid residue. The outcome of these experiments might be influenced by the fact that heterologous expression of CYP71D174 was performed with a chimeric enzyme: to enhance enzyme expression in E. coli, the first 10 N-terminal amino acids were replaced by a modified bovine 17 alphahydroxylase. Thus, final conclusions can first be drawn after heterologous expression of the native gene or investigations in planta. Indeed, in vivo these findings are not reflected, since the essential oil from the P. frutescens variety originally used to clone the gene did neither exhibit E-isopiperitenol nor E-carveol nor traces or derived products thereof (Mau et al. 2010). Recently, five new members of the CYP71Dsubfamily (CYP71D178–182) have been cloned from oregano (Origanum vulgare) and thyme (Thymus vulgaris). Comprehensive studies of CYP71D178, CYP71D180 and CYP71D181—both in vitro and in vivo—demonstrated their role in the biosynthesis of the phenolic monoterpenes thymol and carvacrol. Unlike limonene-hydroxylases of mint species, all cytochromes P450 tested accepted a variety of different monoterpenes as substrates: c-terpinene, a-terpinene, (-)-R-a-phelladrene and (?)-R- as well as (-)-Slimonene. Results achieved rendered possible the prediction of a reaction mechanism that proceeds via an allylic alcohol intermediate instead of p-cymene. With regards to structure–function relationship, sequence comparisons do not indicate prominence of the residue located five positions downstream of the ExxR-motif. Just like CYP71D18, all three cytochromes P450 tested bear a phenylalanine residue at this position. But their product profiles do not 123 16 necessarily correlate to each other or with CYP71D18. Instead, Crocoll and co-workers point at a residue two amino acids downstream of this position with a marked difference among the enzymes. Further investigations including homology modelling and mutational studies are necessary to clarify its significance (Crocoll 2011). Another cytochrome P450 involved in monoterpene metabolism is CYP71A32 (menthofuran synthase) that catalyses conversion of (?)-pulegone to (?)-menthofuran via allylic hydroxylation (Bertea et al. 2001). This reaction can be regarded as unusual, since the oxygen of the furan is not derived from the original carbonyl oxygen but molecular oxygen (Mizutani and Sato 2011). Especially under stressful growth conditions (drought, high temperature, low light intensity), menthofuran concentration in the essential oil of peppermint can reach more than 5 % which results in strong odour and off-colour on storage. Thus, down-regulation of CYP71A32 has been the focus of metabolic engineering in transgenic peppermint plants (Mahmoud and Croteau 2001). Domestication of strawberry (Fragaria ssp.) has led to alterations in fruit flavour and aroma that are mainly characterized by the presence or absence of certain mono- and diterpenoids. Both wild and cultivated strawberries express a pinene hydroxylase (PINH) in ripe fruit and root tissue. Heterologously expressed, this cytochrome P450, FaCYP71AR1, hydroxylates a-pinene and limonene yielding myrtenol and perillyl alcohol, respectively. It is mainly olefinic monoterpenes and myrtenyl acetate that contribute to the volatile profile of wild strawberries (F. vesca). Perillyl alcohol cannot be detected since its precursor is lacking; the terpene synthase (FvPINS) of the wild strawberry only produce linalool, a-pinene, b-myrcene and b-phellandrene, but no limonene (Mau and Croteau 2006). The corresponding gene, however, is defective in cultivated strawberry, and the terpene synthase (FaNES1) catalyses the formation of the sesquiterpenes nerolidol and linalool, thus establishing the volatile profile of these fruits (Aharoni et al. 2004). Finally a cytochrome P450 catalysing an atypical reaction should be mentioned: CYP72A1, secologanin synthase from Catharanthus roseus, involved in terpenoid indole alkaloid biosynthesis. The mechanism of reaction, C–C bond cleavage has been shown to proceed through a radical mechanism (Irmler et al. 2000; Yamamoto et al. 2000), which is more closely described in Mizutani and Sato (2011). First data 123 Phytochem Rev (2015) 14:7–24 suggested that the reaction takes place in the vacuole (Contin et al. 1999), and it was assumed that it is the lack of the proline-rich motif ([P/I]Px[P/G]xP), usually closely located to the N-terminal membrane anchor, that prevents membrane-binding but targets the enzyme to the vacuole instead. This speculation was further supported by the fact that strictosidine synthase, which in a subsequent step conjugates secologanin and tryptamin, is localized in the vacuole (Irmler et al. 2000). Yamamoto et al. (2000), in contrast, demonstrated a tenfold higher enzyme activity in the microsomal fraction of Lonicera japonicus-suspension cultures cells than in the crude extract, thereby indicating that the enzyme is rather membrane-associated. CYP72A1 is atypical in another way: it is the only cytochrome P450 not belonging to CYP71 clan so far to be described being involved in biosynthesis of specialized terpenoids, and thus is derived from a different ancestoral gene than all other cytochrome P450 described in this publication (see Fig. 1). Cytochromes P450 involved in sesquiterpene biosynthesis So far, less than ten cytochromes P450 mediating steps in sesquiterpene biosynthesis have been thoroughly investigated after cloning and heterologous expression. Within these, an unexpected substrate promiscuity as well as flexibility regarding regiospecificity has been observed. One of the first cytochrome P450 involved in sesquiterpene biosynthesis studied was 5-epi-aristolochene 1,3-dihydroxylase (CYP71D20) that plays a decisive role in capsidiol biosynthesis in Nicotiana tabacum (Solanaceae). Since the transformation of 5-epi-aristolochene to capsidiol requires two hydroxylation reactions that are both specific with regards to their regio- and stereospecificity, at first, catalysis through a single enzyme was not expected. Also, the mechanism of reaction remained unknown for a long time since the existence of monohydroxylated intermediates could not be proven (Ralston et al. 2001). Instead, it was suggested that the monohydroxylated intermediate rotated within the active site. Study of CYP71D20’s structure–function relationship, accomplished by molecular modelling and targeted mutation, have eventually demonstrated that the preferred reaction order of hydroxylation is at Phytochem Rev (2015) 14:7–24 C-1 followed by C-3. It remains to be shown whether the intermediate is released in between the reactions (Takahashi et al. 2005). Coupled assays with CYP71D20 and the sesquiterpene synthases germacrene A synthase and premnaspirodiene synthase, respectively, revealed that hydroxylation of germacrene A proceeds at two different positions, whereas premnaspirodiene is hydroxylated twice at position 2, the latter resulting in the corresponding ketone, solavetivone (Greenhagen et al. 2003). In planta data are missing, but needed in order to demonstrate, whether the outcome of these experiments indeed reflect endogenous enzyme activities or whether the observations were affected by the artificial environment of the in vitro-tests. Investigations into the relationship between structure and function of CYP71D20 focused on two amino acids: serine368 and isoleucine486. Ser368 is located 5 amino acids after the highly conserved ExxR-motif, a position previously demonstrated to be critical for substrate-enzyme interaction (cp. CYP71D18). Ile486 occupies a position in SRS 6 that has been found to be highly variable. Targeted-mutation of these residues indicated that both positions are crucial for regio- and stereospecificity as well as regulation of the catalysed reactions (Takahashi et al. 2005). Conclusions achieved in these studies prove incoherent when CYP71D55, premnaspirodiene oxygenase from Hyoscyamus muticus (Solanaceae), was examined. Besides its native substrate premnaspirodiene, CYP71D55 is capable of hydroxylating valencene, 5-epi-eremophilene, and 5-epi-aristolochene. The C-2 atom of premnaspirodiene is hydroxylated twice yielding solavetivone. In contrast, 5-epi-eremophilene and 5-epi-aristolochene are only hydroxylated once. Comparison of CYP71D20 and CYP71D55 revealed high identity on amino acid level. Within domains relating to SRS 5 and 6, differences in only 4 amino acids were observed. Comprehensive mutational studies on these 4 residues were conducted both in CYP71D20 and CYP71D55. The experiments demonstrated that these 4 residues indeed influence catalytic efficiency but do not lead to alternation of regio- or stereospecificity, the latter was evident in case of 5-epi-aristolochene that is hydroxylated at different positions by CYP71D20 and CYP71D55, respectively (see Table 2). The regiospecificity of CYP71D55 appears independent of the overall hydrocarbon scaffold (spirovetivane vs. eremophilane). 17 Stereochemistry, however, seems to be best predicted by the orientation of the vicinal methyl at C-14 and C-15 instead of the position of the double bond in the A or B ring, since the vicinal methyl groups guides the cytochrome P450 in the opposite stereochemical direction. For the reaction mechanism, it is likely that the two successive hydroxylations are undertaken independently, but do not necessarily depend on a dissociation of the first substrate (Takahashi et al. 2007). Since only solavetivol but not nootkatol is converted to the corresponding ketone, it can be presumed that the active site is restricted, although further studies are needed in order to elucidate these promiscuous enzymes. Within cotton plants (Gossypium arboreum, Malvaceae), most sesquiterpenoids derive from d-cadinene. One of these is gossypol, a polyphenol with high antioxidative activity. In the plant, gossypol serves as a defence compound by causing infertility in most male animals including humans. Therefore, gossypol was tested as a contraceptive for men (Coutinho 2002). Apart from the sesquiterpene synthase that catalyses formation of cadinene, only an O-methyl transferase and a cytochrome P450 involved in gossypol’s biosynthesis have been described. CYP706B1 (part of the CYP71 clan) catalyses hydroxylation of d-cadinene at C8 (Luo et al. 2001). To date, CYP706B1 is the only member of the CYP706-family with assigned function (Nelson and Werck-Reichhart 2011). CYP706B1’s catalytical activity was studied towards compounds present in cotton glands (a-humulene and a-copaene) and structurally related compounds (a-cubebene and amuurolene). All substrates were hydroxylated once with the exception of a-copaene, which was not transformed. In several cases, generation of up to four products was reported; exact structures of the products have not been published (Wang and Essenberg 2010). The cytochrome P450 CYP71BA1 from Zingiber zerumbet (Zingiberaceae) catalyses the conversion of a-humulene to 8-hydroxy-a-humulene in the zerumbone biosynthesis. After heterologous expression of the gene in yeast, formation of this single product was described (Yu et al. 2011). Therefore, it was proposed that the subsequent and final step of zerumbone biosynthesis was mediated by an alcohol dehydrogenase, whose elucidation was reported in a recent publication (Okamoto et al. 2011). Sesquiterpene lactones are characteristic products of species in the large plant families Asteraceae and 123 123 71AV8 GAO Artemisia annua 71AV1 (Asteraceae) Cichorium intybus (all: Asteraceae) Barnadesia spinosa Helianthus annuus OH H O HO O It also catalyses hydroxylation of germacrene A and amorphadiene-4,11-diene affording artemisinic acid and germacrene A acid, respectively (see CYP71AV1 and GAO) germacrene A acid HQ166835 GU256647 GU256646 GU256645 Saussurea costus artemisinic acid OH O GU256644 H O Cankar et al. (2011) Nguyen et al. (2010) Ro et al. (2008), Teoh et al. (2006) DQ315671 Cichorium intybus germacrene A OH References Database number GU198171 amorpha-4,11-diene Catalysed eaction Lactuca sativa (Asteraceae) Plant origin Cytochrome P450 Table 2 Cytochrome P450 genes involved in sesquiterpene biosynthesis 18 Phytochem Rev (2015) 14:7–24 71D20 71BL3 71BL2 71BL1 Zingiber zerumbet 71BA1 (Solanaceae) Nicotiana tabacum (Asteraceae) Cichorium intybus (Asteraceae) Lactuca sativa (Asteraceae) Helianthus annuus (Zingiberaceae) Plant origin Cytochrome P450 Table 2 continued Germacrene A and spirodiene are also hydroxylated, but only with (very) low efficiency Catalysed eaction AF368376 JF816041 HQ439599 Greenhagen et al. (2003), Ralston et al. (2001), Takahashi et al. (2005) Liu et al. (2011) Ikezawa et al. (2011) Ikezawa et al. (2011) Yu et al. (2011) AB331234 HQ439587 References Database number Phytochem Rev (2015) 14:7–24 19 123 123 706B1 Hyoscyamus muticus 71D55 (Malvaceae) Gossypium arboreum (Solanaceae) Plant origin Cytochrome P450 Table 2 continued Additional substrates are (-)-d-cadinene, (-)-a-cubebene, (-)-a-muurolene, a-humulene, the structure of the respective products has not been described Catalysed eaction Luo et al. (2001), Wang and Essenberg (2010) Takahashi et al. (2007) EF569601 AF332974 References Database number 20 Phytochem Rev (2015) 14:7–24 Phytochem Rev (2015) 14:7–24 Apiaceae. Many of them are not only of importance for the plants, but also exhibit social and economic value to mankind. Artemisinin, helenanin, matricin and thapsigargin exhibit curative effects that are applied for the treatment of diseases both in conventional and traditional medicine (Drew et al. 2009; Ramadan et al. 2006; Ro et al. 2006), nootkatone can be used as flavour and fragrance ingredient by the food industry (Berry et al. 1967), to mention only a few of them. Thus, interest in the biosynthesis of these compounds is high. The discovery of CYP71AV1, amorpha-4,11-diene oxidase of Artemisia annua, rendered possible the biotechnological synthesis of an artemisinin precursor for the first time. Catalysed by the enzyme’s activity, amorpha-4,11-diene is transformed into artemisinic acid in three consecutive hydroxylation steps (Ro et al. 2006). Even though artemisinic acid does not serve as artemisinin precursor in planta (Bertea et al. 2005; Teoh et al. 2009), it is a valuable compound for semisynthetic artemisinin production. CYP71AV1 has additionally been studied in planta. Examinations of its expression in Nicotiana species have been published recently by two groups. In N. benthamiana, co-expression of amorpha4,11-diene synthase (ADS) and CYP71AV1 led to the formation of artemisinic acid, which was partly recovered as diglucoside (van Herpen et al. 2010). The group of Brodelius, on the other hand, only obtained amorpha4,11-diene and artemisinic alcohol when expressing the same enzymes in N. tabacum. This finding was confirmed studying the enzymes in Artemisia annua (Olofsson et al. 2011). Studies on expression and product formation of the two dehydrogenases (alcohol and aldehyde) along with CYP71AV1 demonstrated that CYP71AV1 can catalyse the production of both the alcohol and the aldehyde, but also that several endogenous A. annua dehydrogenases can catalyse the production of the acid and the aldehyde (Li et al. 2012; Olofsson et al. 2011). Subsequently, CYP71AV-genes were isolated from seven more Asteraceae species (CYP71AV2–8). Indeed, the CYP71AV-subfamily is conserved in all major aster subfamilies and might even be highly specific for sesquiterpene lactone biosynthesis (Teoh et al. 2006). With the exception of CYP71AV1, all other subfamily members are germacrene A oxidases (GAO) with exceptional broad substrate specificity. In vitro, GAOs have been shown to mediate hydroxylation of amongst others germacrene A, valencene and amorpha-4,11-diene to the corresponding acids 21 (Cankar et al. 2011; Nguyen et al. 2010). Since hydroxylation does not proceed regiospecifically, the hypothesis of two distinct categories of sesquiterpene modifying cytochromes P450 was disproved: a division between those acting on the A-ring like CYP71D55 and CYP71D20 and those that act on allylic C12 as CYP71AV1 cannot further be sustained. Based on these observations, CYP71AV-subfamily has been chosen for the study of enzyme evolution. It has been assumed that substrate promiscuity provides selective advantage for an enzyme, allowing optimization of one specific function under specific selection pressure as observed in Artemisia annua: mutations both in its terpene synthase as well as GAO gave rise to unique artemisinin biosynthesis (Nguyen et al. 2010). Members of a novel CYP71-subfamily mediate hydroxylation of germacrene A acid at positions adjacent to the carboxy group. CYP71BL2 from Lactuca sativa (Ikezawa et al. 2011) and CYP71BL3 from Cichorium intybus (Liu et al. 2011) specifically catalyse 6a-hydroxylation. Subsequently, the intermediate undergoes spontaneous lactone formation that results in costunolide. Homologues of CYP71BL2 have been found in the EST databases of all major Asteraceae subfamilies except of the Helianthae tribe implying that costunolide biosynthesis is conserved in most Asteraceae genera. Instead, Helianthae possess ESTs highly homologue to CYP71BL1, an 8b-hydroxylase that has been isolated from Helianthus annuus. In case of 8bhydroxylation, no spontaneous lactone formation has been observed due to the physiochemical properties of the molecule, which show that the lactone 8,12guaianolides need a yet non-disclosed enzyme in order to be formed. So far, comparative genomics analysis suggests that Asteraceae either possess a homologue to the 6a- or the 8b-hydroxylase, but not both of them. This does, however, not correlate with existence or absence of sesquiterpene lactones of the costunolidetype (Ikezawa et al. 2011). References Aharoni A, Giri AP, Verstappen FW et al (2004) Gain and loss of fruit flavor compounds produced by wild and cultivated strawberry species. Plant Cell 16(11):3110–3131 Ashour M, Wink M, Gershenzon J (2010) Biochemistry of terpenoids: monoterpenes, sesquiterpenes and diterpenes. Annu Plant Rev 40:258–303 123 22 Bak S, Beisson F, Bishop G et al (2011) Cytochromes P450. The Arabidopsis Book e0144 Berger RG (ed) (2007) Flavours and fragrances chemistry, bioprocessing and sustainability, vol XVI. Springer, Heidelberg Bernhardt R (2006) Cytochromes P450 as versatile biocatalysts. J Biotechnol 124(1):128–145 Berry R, Wagner CJ, Moshonas MG (1967) Flavor studies of nootkatone in grapefruit juice. J Food Sci 32:75–78 Bertea CM, Schalk M, Karp F et al (2001) Demonstration that menthofuran synthase of mint (Mentha) is a cytochrome P450 monooxygenase: cloning, functional expression, and characterization of the responsible gene. Arch Biochem Biophys 390(2):279–286 Bertea CM, Freije JR, van der Woude H et al (2005) Identification of intermediates and enzymes involved in the early steps of artemisinin biosynthesis in Artemisia annua. Planta Med 71(1):40–47 Bohlmann J, Keeling CI (2008) Terpenoid biomaterials. Plant J 54(4):656–669 Bohlmann J, Meyer-Gauen G, Croteau R (1998) Plant terpenoid synthases: molecular biology and phylogenetic analysis. PNAS 95(8):4126–4133 Bozak KR, O’Keefe DP, Christoffersen RE (1992) Expression of a ripening-related Avocado (Persea americana) cytochrome P450 in yeast. Plant Phys 100:1976–1981 Buckingham J (2011) Dictionary of natural products. http://www. chemnetbase.com Cankar K, van Houwelingen A, Bosch D et al (2011) A chicory cytochrome P450 mono-oxygenase CYP71AV8 for the oxidation of (?)-valencene. FEBS Lett 585(1):178–182 Clark IM, Forde BG, Hallahan DL (1997) Spatially distinct expression of two new cytochrome P450s in leaves of Nepeta racemosa: identification of a trichome-specific isoform. Plant Mol Biol 33(5):875–885 Collu G, Unver N, Peltenburg-Looman AM et al (2001) Geraniol 10-hydroxylase, a cytochrome P450 enzyme involved in terpenoid indole alkaloid biosynthesis. FEBS Lett 508(2):215–220 Contin A, Van der Heijden R, Verpoorte R (1999) Accumulation of loganin and secologanin in vacuoles from suspension cultured Catharanthus roseus cells. Plant Sci 147:177–183 Coutinho EM (2002) Gossypol: a contraceptive for men. Contraception 65(4):259–263 Cragg GM, Kingston DGI, Newman DJ (2011) Anticancer agents from natural products, 2nd edn. CRC Press, Boca Raton Crocoll C (2011) Biosynthesis of the phenolic monoterpenes, thymol and carvacrol, by terpene synthases and cytochrome P450s in oregano and thyme. PhD Thesis, Friedrich-Schiller-Universität Daviet J, Schalk M (2010) Biotechnology in plant essential oil production: progress and perspective in metabolic engineering of the terpene pathway. Flav Frag J 25:123–127 Devi BSR, Kim YJ, Sathiyamoorthy S et al (2011) Classification and characterization of putative cytochrome P450 genes from Panax ginseng C. A. Meyer. Biochemistry 76(12): 1347–1359 Dictionary of Natural Products (2011) Chapman and Hall/CRC. http://www.chemnetbase.com. Accessed Jan 2012 123 Phytochem Rev (2015) 14:7–24 Drew DP, Krichau N, Reichwald K et al (2009) Guaianolides in apiaceae: perspectives on pharmacology and biosynthesis. Phytochem Rev 8(3):581–599 El-Sayed M, Verpoorte R (2007) Catharanthus terpenoid indole alkaloids: biosynthesis and regulation. Phytochem Rev 6(2–3):277–305 Evans WC (2009) Trease and evans: pharmacognosy, 16th edn. Elsevier, London Gershenzon J, Dudareva N (2007) The function of terpene natural products in the natural world. Nat Chem Biol 3(7):408–414 Gershenzon J, McConkey ME, Croteau RB (2000) Regulation of monoterpene accumulation in leaves of peppermint. Plant Phys 122(1):205–214 Graham SE, Peterson JA (1999) How similar are P450s and what can their differences teach us? Arch Biochem Biophys 369(1):24–29 Greenhagen BT, Griggs P, Takahashi S et al (2003) Probing sesquiterpene hydroxylase activities in a coupled assay with terpene synthases. Arch Biochem Biophys 409(2):385–394 Guengerich FP (2001) Common and uncommon cytochrome P450 reactions related to metabolism and chemical toxicity. Chem Res Toxicol 14(6):611–650 Hallahan DL, Nugent JH, Hallahan BJ et al (1992) Interactions of Avocado (Persea americana) Cytochrome P-450 with Monoterpenoids. Plant Phys 98(4):1290–1297 Hallahan DL, Lau SM, Harder PA et al (1994) Cytochrome P-450-catalysed monoterpenoid oxidation in catmint (Nepeta racemosa) and avocado (Persea americana); evidence for related enzymes with different activities. Biochim Biophys Acta 1201(1):94–100 Hamberger B, Bak S (2013) Plant P450s as versatile drivers for evolution of species-specific chemical diversity. Phil Trans R Soc B 368(1612):1471–2970 Hamdane D, Zhang H, Hollenberg P (2008) Oxygen activation by cytochrome P450 monooxygenase. Photosynth Res 98(1–3):657–666 Hasemann CA, Kurumbail RG, Boddupalli SS et al (1995) Structure and function of cytochromes P450: a comparative analysis of three crystal structures. Structure 3(1):41–62 Haudenschild C, Schalk M, Karp F et al (2000) Functional expression of regiospecific cytochrome P450 limonene hydroxylases from mint (Mentha spp.) in Escherichia coli and saccharomyces cerevisiae. Arch Biochem Biophys 379(1):127–136 Ikezawa N, Gopfert JC, Nguyen DT et al (2011) Lettuce costunolide synthase (CYP71BL2) and its homolog (CYP71BL1) from sunflower catalyze distinct regio- and stereoselective hydroxylations in sesquiterpene lactone metabolism. J Biol Chem 286(24):21601–21611 Irmler S, Schroder G, St-Pierre B et al (2000) Indole alkaloid biosynthesis in Catharanthus roseus: new enzyme activities and identification of cytochrome P450 CYP72A1 as secologanin synthase. Plant J 24(6):797–804 Kaltenbach M, Schroder G, Schmelzer E et al (1999) Flavonoid hydroxylase from Catharanthus roseus: cDNA, heterologous expression, enzyme properties and cell-type specific expression in plants. Plant J 19(2):183–193 Li X, Ma D, Chen J et al (2012) Biochemical characterization and identification of a cinnamyl alcohol dehydrogenase from Artemisia annua. Plant Sci 193–194:85–95 Phytochem Rev (2015) 14:7–24 Liu Q, Majdi M, Cankar K et al (2011) Reconstitution of the costunolide biosynthetic pathway in yeast and Nicotiana benthamiana. PLoS ONE 6(8):e23255 Luo P, Wang YH, Wang GD et al (2001) Molecular cloning and functional identification of (?)-delta-cadinene-8-hydroxylase, a cytochrome P450 mono-oxygenase (CYP706B1) of cotton sesquiterpene biosynthesis. Plant J 28(1):95–104 Lupien S, Karp F, Wildung M et al (1999) Regiospecific cytochrome P450 limonene hydroxylases from mint (Mentha) species: cDNA isolation, characterization, and functional expression of (-)-4S-limonene-3-hydroxylase and (-)-4Slimonene-6-hydroxylase. Arch Biochem Biophys 368(1): 181–192 Mahmoud SS, Croteau RB (2001) Metabolic engineering of essential oil yield and composition in mint by altering expression of deoxyxylulose phosphate reductoisomerase and menthofuran synthase. PNAS 98(15):8915–8920 Mau CJD, Croteau R (2006) Cytochrome P450 oxygenases of monoterpene metabolism. Phytochem Rev 5:373–383 Mau CJ, Karp F, Ito M et al (2010) A candidate cDNA clone for (-)-limonene-7-hydroxylase from Perilla frutescens. Phytochemistry 71(4):373–379 Miller B, Madilao LL, Ralph S et al. (2005) Insect-induced conifer defense. White pine weevil and methyl jasmonate induce traumatic resinosis, de novo formed volatile emissions, and accumulation of terpenoid synthase and putative octadecanoid pathway transcripts in Sitka spruce. Plant Phys 137(1):369–382 Mizutani M (2012) Impacts on diversifation of cytochrome P450 on plant metabolism. Biol Pharm Bull 35(6):824–832 Mizutani M, Sato F (2011) Unusual P450 reactions in plant secondary metabolism. Arch Biochem Biophys 507(1): 194–203 Mizutani M, Ward E, Ohta D (1997) Plant geraniol/nerol 10-hydroxylase and DNA coding therefor. US Patent Nelson DR (2011) Progress in tracing the evolutionary paths of cytochrome P450. Biochim Biophys Acta 1814(1):14–18 Nelson D (2012) Personal communication Nelson D, Werck-Reichhart D (2011) A P450-centric view of plant evolution. Plant J 66(1):194–211 Nguyen DT, Gopfert JC, Ikezawa N et al (2010) Biochemical conservation and evolution of germacrene a oxidase in asteraceae. J Biol Chem 285(22):16588–16598 O’Connor SE, Maresh JJ (2006) Chemistry and biology of monoterpene indole alkaloid biosynthesis. Nat Prod Rep 23(4):532–547 O’Connor SE, McCoy E (2006) Chapter one Terpene indole alkaloid biosynthesis. In: John TR (ed) Integrative plant biochemistry. Recent advances in phytochemistry, vol 40. Elsevier, Amsterdam, pp 1–22 Okamoto S, Yu F, Harada H et al (2011) A short-chain dehydrogenase involved in terpene metabolism from Zingiber zerumbet. FEBS J 278(16):2892–2900 Olofsson L, Engstrom A, Lundgren A et al (2011) Relative expression of genes of terpene metabolism in different tissues of Artemisia annua L. BMC Plant Biol 11(1):45 Omura T, Sato R (1964) The carbon monoxide-binding pigment of liver microsomes. I. Evidence for its hemoprotein nature. J Biol Chem 239:2370–2378 Oudin A, Mahroug S, Courdavault V et al (2007) Spatial distribution and hormonal regulation of gene products from 23 methyl erythritol phosphate and monoterpene-secoiridoid pathways in Catharanthus roseus. Plant Mol Biol 65(1–2): 13–30 Peterson JA, Graham SE (1998) A close family resemblance: the importance of structure in understanding cytochromes P450. Structure 6(9):1079–1085 Ralston L, Kwon ST, Schoenbeck M et al (2001) Cloning, heterologous expression, and functional characterization of 5-epi-aristolochene-1,3-dihydroxylase from tobacco (Nicotiana tabacum). Arch Biochem Biophys 393(2): 222–235 Ramadan M, Goeters S, Watzer B et al (2006) Chamazulene carboxylic acid and matricin: a natural profen and its natural prodrug, identified through similarity to synthetic drug substances. J Nat Prod 69(7):1041–1045 Ro DK, Paradise EM, Ouellet M et al (2006) Production of the antimalarial drug precursor artemisinic acid in engineered yeast. Nature 440(7086):940–943 Ro DK, Ouellet M, Paradise EM et al (2008) Induction of multiple pleiotropic drug resistance genes in yeast engineered to produce an increased level of anti-malarial drug precursor, artemisinic acid. BMC Biotechnol 8:83 Robineau T, Batard Y, Nedelkina S et al (1998) The chemically inducible plant cytochrome P450 CYP76B1 actively metabolizes phenylureas and other xenobiotics. Plant Phys 118(3):1049–1056 Schalk M, Croteau R (2000) A single amino acid substitution (F363I) converts the regiochemistry of the spearmint (-)limonene hydroxylase from a C6- to a C3-hydroxylase. PNAS 97(22):11948–11953 Schuler MA, Werck-Reichhart D (2003) Functional genomics of P450s. Annu Rev Plant Biol 54(1):629–667 Seifert A, Pleiss J (2009) Identification of selectivity-determining residues in cytochrome P450 monooxygenases: a systematic analysis of the substrate recognition site 5. Proteins 74(4):1028–1035 Simonsen HT, Drew DP, Lunde C (2009) Perspectives on using Physcomitrella Patens as an alternative production platform for Thapsigargin and other Terpenoid drug candidates. Perspect Med Chem 3:1–6 Sirim D, Widmann M, Wagner F et al (2010) Prediction and analysis of the modular structure of cytochrome P450 monooxygenases. BMC Struct Biol 10:34 Sono M, Roach MP, Coulter ED et al (1996) Heme-containing oxygenases. Chem Rev 96(7):2841–2888 Sung PH, Huang FC, Do YY et al (2011) Functional expression of geraniol 10-hydroxylase reveals its dual function in the biosynthesis of terpenoid and phenylpropanoid. J Agric Food Chem 59(9):4637–4643 Takahashi S, Zhao Y, O’Maille PE et al (2005) Kinetic and molecular analysis of 5-epiaristolochene 1,3-dihydroxylase, a cytochrome P450 enzyme catalyzing successive hydroxylations of sesquiterpenes. J Biol Chem 280(5): 3686–3696 Takahashi S, Yeo YS, Zhao Y et al (2007) Functional characterization of premnaspirodiene oxygenase, a cytochrome P450 catalyzing regio- and stereo-specific hydroxylations of diverse sesquiterpene substrates. J Biol Chem 282(43): 31744–31754 Teoh KH, Polichuk DR, Reed DW et al (2006) Artemisia annua L. (Asteraceae) trichome-specific cDNAs reveal 123 24 CYP71AV1, a cytochrome P450 with a key role in the biosynthesis of the antimalarial sesquiterpene lactone artemisinin. FEBS Lett 580(5):1411–1416 Teoh KH, Polichuk DR, Reed DW et al (2009) Molecular cloning of an aldehyde dehydrogenase implicated in artemisinin biosynthesis in Artemisia annua. Botany 87(6): 635–642 van Herpen TW, Cankar K, Nogueira M et al (2010) Nicotiana benthamiana as a production platform for artemisinin precursors. PLoS ONE 5(12):e14222 Wang YH, Essenberg M (2010) Inhibitor and substrate activities of sesquiterpene olefins toward (?)-[delta]-cadinene8-hydroxylase, a cytochrome P450 monooxygenase (CYP706B1). Phytochemistry 71(16):1825–1831 Wang J, Liu Y, Cai Y et al (2010) Cloning and functional analysis of geraniol 10-hydroxylase, a cytochrome P450 from Swertia mussotii Franch. Biosci Biotechnol Biochem 74(8):1583–1590 Werck-Reichhart D, Feyereisen R (2000) Cytochromes P450: a success story. Genome Biol 1(6):REVIEWS3003 123 Phytochem Rev (2015) 14:7–24 Wust M, Croteau RB (2002) Hydroxylation of specifically deuterated limonene enantiomers by cytochrome p450 limonene-6-hydroxylase reveals the mechanism of multiple product formation. Biochemistry 41(6):1820–1827 Wust M, Little DB, Schalk M et al (2001) Hydroxylation of limonene enantiomers and analogs by recombinant (-)limonene 3- and 6-hydroxylases from mint (Mentha) species: evidence for catalysis within sterically constrained active sites. Arch Biochem Biophys 387(1):125–136 Yamamoto H, Katano N, Ooi A et al (2000) Secologanin synthase which catalyzes the oxidative cleavage of loganin into secologanin is a cytochrome P450. Phytochemistry 53(1):7–12 Yu F, Okamoto S, Harada H et al (2011) Zingiber zerumbet CYP71BA1 catalyzes the conversion of alpha-humulene to 8-hydroxy-alpha-humulene in zerumbone biosynthesis. Cell Mol Life Sci 68(6):1033–1040 Zwenger S, Basu C (2008) Plant terpenoids: applications and future potentials. Biotechnol Mol Biol Rev 3(1):1–7